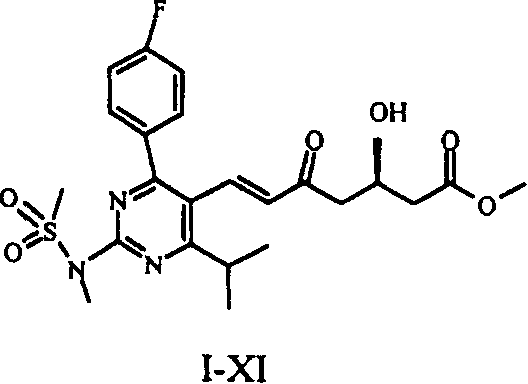
Method for preparing Rosuvastatin Calcium and key intermediate
A technology for rosuvastatin calcium and intermediates, which is applied in the field of preparation of key intermediates, and can solve problems such as increased impurity content, affecting the completeness of stereoselective reactions, and large content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
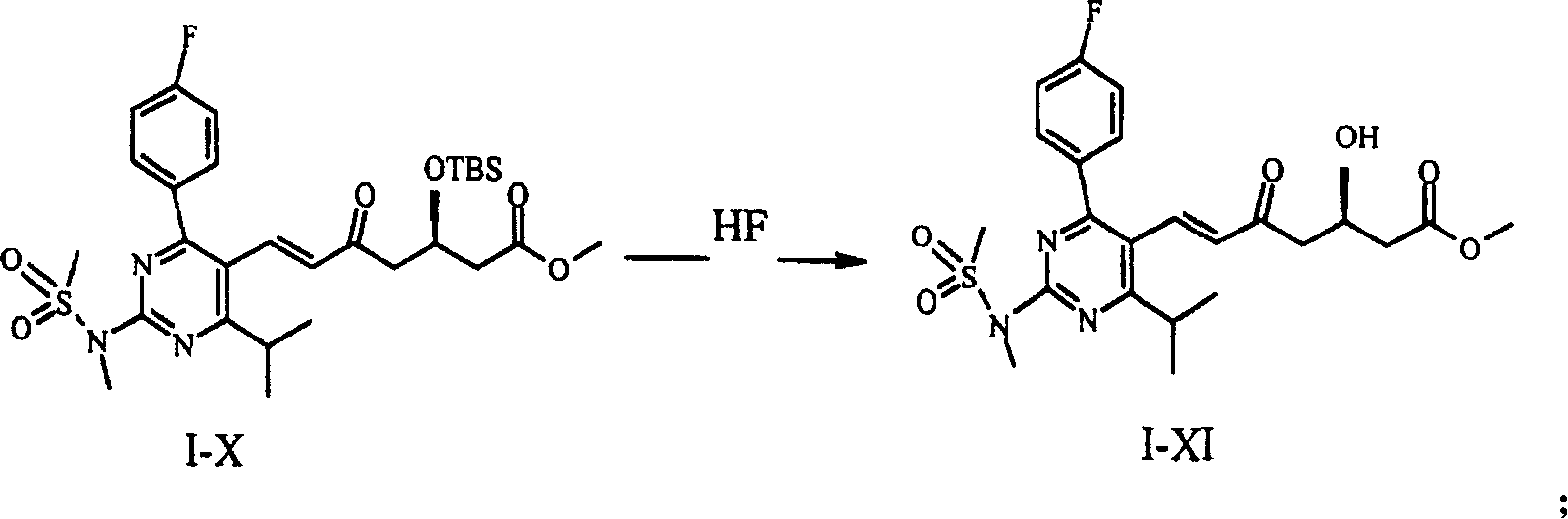
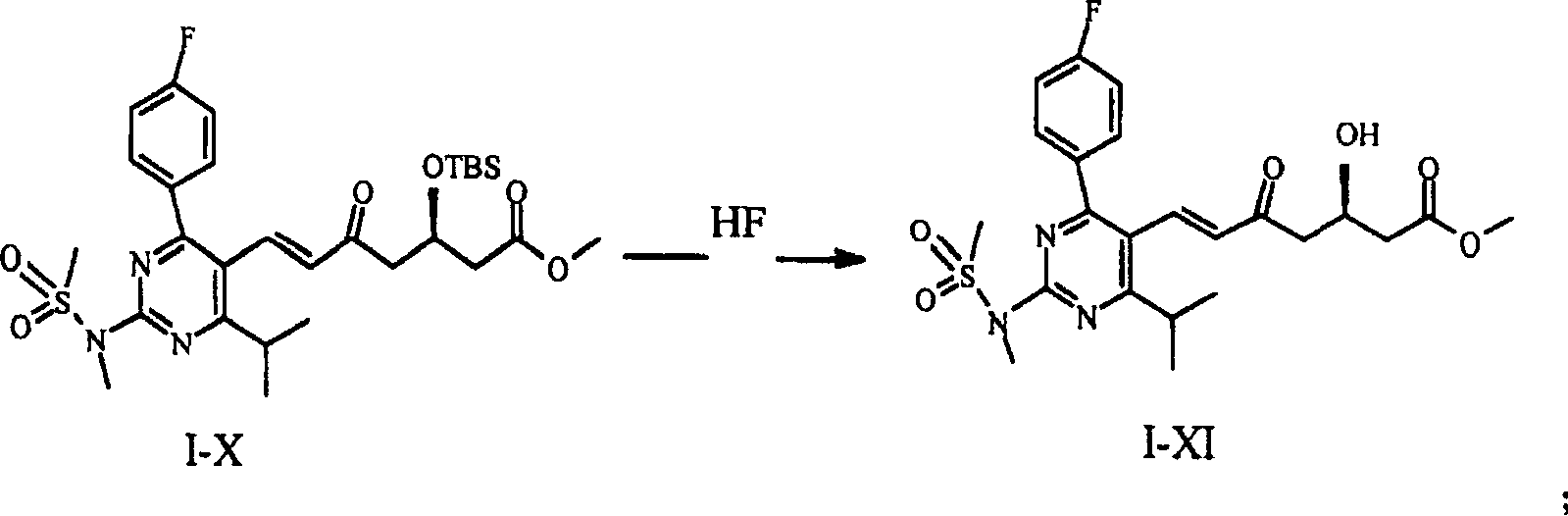
[0028] Compounds I-X were prepared as described in EP 0,521,471 A1. Weigh 16 g of compound I-X and dissolve it in 100 ml of acetonitrile to obtain solution 1. Under ice-bath conditions, 20ml of 48% (w / v) HF aqueous solution was added to 380ml of acetonitrile to obtain solution 2. Solution 1 was added dropwise to solution 2 in an ice bath. After the dropwise addition was completed, it was heated to room temperature and stirred for 1.5 hr. Then use Na 2 CO 3 Neutralize and extract with ether. The organic layer was separated and harvested, washed with sodium chloride, dried, and the solvent was evaporated under reduced pressure to obtain 14.6 ml of a concentrated solution. To the concentrated solution was added 14.6 ml of toluene / diethyl ether 3:2 (v / v) mixture, and the ratio of concentrated solution:mixed solution (w / v) was 1:1. Stir at room temperature until dissolved. Cool to 0-4 ° C to crystallize. Filter the reaction solution after crystallization, and spot the filtr...
Embodiment 2
[0031] 30 g of compound I-X was dissolved in 800 ml of acetonitrile, 20 ml of 48% (w / v) HF solution was added, and reacted at room temperature for 10 hr. Add NaHCO under ice-salt cooling 3 , adjust the pH to 7, and extract with ether. The organic layer was separated and harvested, washed with saturated brine, washed with anhydrous Na 2 SO 4 Dry, filter, and distill off the solvent under reduced pressure to obtain 26.8 g of an oily concentrate. Add 40.2ml of toluene / diethyl ether 2:1 (v / v) mixture to the concentrated solution, the ratio of concentrated solution:mixed solution (w / v) is 2:3, stir at room temperature until dissolved. Cool to 0-4 ° C to fully crystallize. After filtration, the filter cake was vacuum-dried to obtain 22.5 g of compound I-XI in the form of yellow crystals. As mentioned above, the purity was 98.8% as determined by HPLC.
[0032] HNMR (CDCl 3 )δ: 1.28(d, 6H), 2.52(m, 2H), 2.73(m, 2H), 3.36(hept, 1H), 3.51(s, 3H), 3.59(s, 3H), 3.72(s, 3H ), 4.48 ...
Embodiment 3
[0034] 20 g of compound I-X was dissolved in 125 ml of acetonitrile to obtain solution 1. Under ice-bath conditions, 25ml of 48% (w / v) HF aqueous solution was added to 475ml of acetonitrile to obtain solution 2. Solution 1 was added dropwise to solution 2 in an ice bath, heated to room temperature after the dropwise addition, and stirred for 1.5 hr. use Na 2 CO 3 Neutralize and extract with ether. The organic layer was separated and harvested, washed with sodium chloride, dried, and the solvent was evaporated under reduced pressure to obtain 18.3 g of a concentrated solution. Add 27.5ml of toluene:diethyl ether 3:1 (v / v) mixture to the concentrated solution, the ratio of concentrated solution:mixed solution (w / v) is 1:1.5, and dissolve at room temperature. Then cool to 0-4 ° C to fully crystallize. After filtration, the filter cake was vacuum-dried to obtain compound I-XI in the form of yellow crystals, 16.1 g. As mentioned above, the purity was 98.9% as determined by HP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com