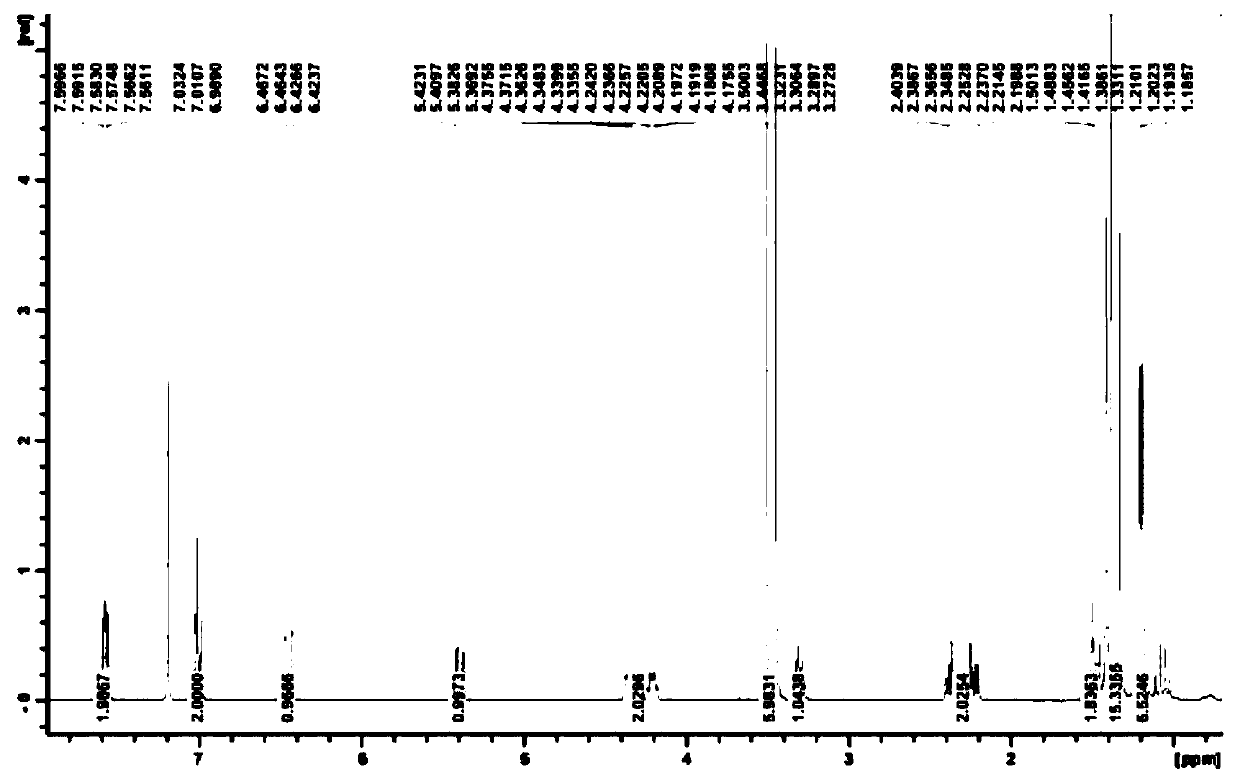
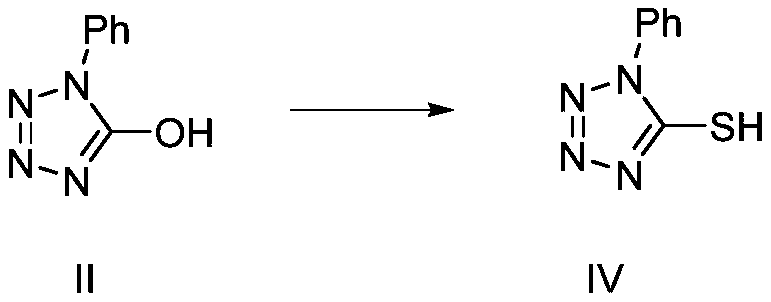
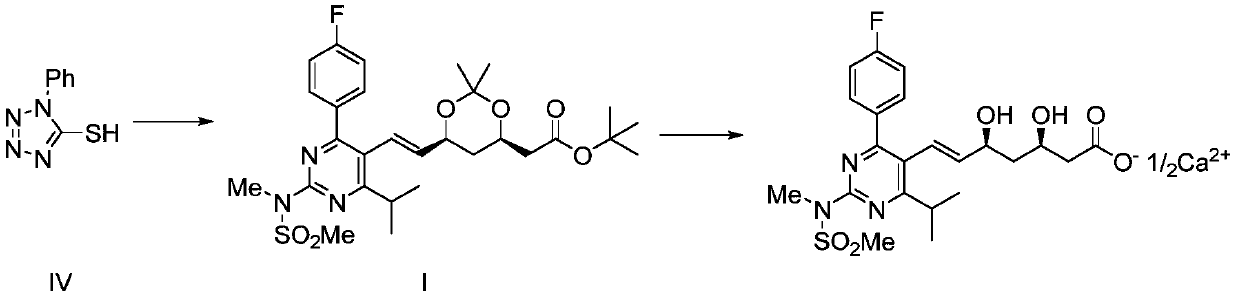
Recycling method of 1-phenyl-5-hydroxytetrazole
A technology of hydroxytetrazolium and mercaptotetrazolium, which is applied in the field of recycling and utilization of 1-phenyl-5-hydroxytetrazolium, and achieves the effects of cost saving, three waste reduction and production cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of 1-phenyl-5-chlorotetrazolium:
[0053] Put 20 grams (0.123 mol) of 1-phenyl-5-hydroxytetrazolium in the reaction flask, add 100 milliliters of toluene, add 11.6 grams of phosphorus pentachloride (0.056 mol), heat to reflux for reaction, and react for 10 hours. The reaction is complete. After removing excess thionyl chloride and solvent under reduced pressure, the crude product was recrystallized once by ethanol to obtain 15.6 g of product with a yield of 70%.
[0054] Synthesis of 1-phenyl-5-mercaptotetrazolium:
[0055] Add 15 g (0.083 mol) of 1-phenyl-5-chlorotetrazolium, add 60 ml of absolute ethanol, then add 12.2 g (0.16 mol) of thiourea, heat to 60° C. under thorough stirring, and react for 10 hours. Cool to 30°C, add 6 grams of solid sodium hydroxide, then slowly raise the temperature to 50°C, and react for 3 hours.
[0056] Cool to room temperature, add 30 ml of water to the reaction system, remove the solvent under reduced pressure, add 50 ml of d...
Embodiment 2
[0059] Put 20 grams (0.123 mol) of 1-phenyl-5-hydroxy tetrazolium in the reaction flask, add 43.8 grams (0.369 mol) of thionyl chloride, add 0.5 milliliters of DMF and 70 milliliters of toluene, heat to reflux, and react In 9 hours, the reaction was complete. After removing excess thionyl chloride under reduced pressure, 22 g of crude product were obtained. Add 10 ml of absolute ethanol and depressurize to dryness without further purification.
[0060] The above solid was directly added to 80 ml of absolute ethanol, then 15 g (0.197 mol) of thiourea was added, heated to 60° C. under thorough stirring, and reacted for 9 h. Cool to 25°C, add 9 grams of solid sodium hydroxide, then slowly raise the temperature to 50°C, and react for 3 hours.
[0061] Cool to room temperature, add 50 ml of water to the reaction system, remove the solvent under reduced pressure, add 100 ml of dichloromethane to extract and remove organic impurities, slowly add 3 mol / L hydrochloric acid to the wat...
Embodiment 3
[0063] Add 15 g (0.093 mol) of 1-phenyl-5-hydroxytetrazolium, add 100 ml of carbon tetrachloride, and 21.5 g (0.05 mol) of phosphorus pentabromide, heat to reflux, and react for 8 hours. After the reaction was completed, the reaction system was poured into 200 grams of ice water. Stirring, liquid separation, the aqueous phase was extracted twice with dichloromethane, 100 ml each time, the organic phases were combined, washed once with 50 ml of sodium bicarbonate solution, washed once with 50 ml of water, dried, concentrated to dryness under reduced pressure, 16.2 g of solid was obtained with a yield of 77.7%. The crude product was recrystallized once by ethanol and set aside.
[0064] Add 15.8 grams of 1-phenyl-5-bromotetrazolium (0.07mol) to 80 milliliters of absolute ethanol, add 8.2 grams (0.108mol) of thiourea, heat to 60°C, react for 9 hours, and cool to 25°C , add 5 grams of solid sodium hydroxide, then slowly raise the temperature to 50°C, and react for 3 hours.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com