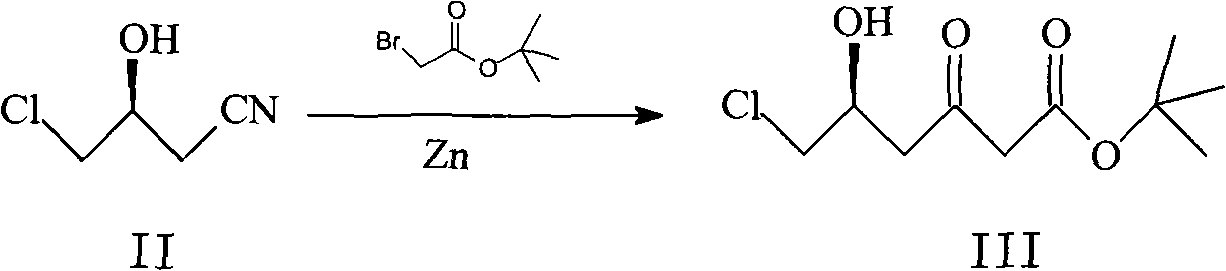Synthetic method of key intermediate of rosuvastatin calcium side chain
A technology of rosuvastatin calcium and its intermediates, which is applied in the field of preparation of blood lipid-lowering drugs, can solve the problems of low yield of side chain and mother nucleus, long synthetic route, instability, etc., and achieve convenient industrial production and high product yield High, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 (S)-6-chloro-5-hydroxyl-3-oxohexanoic acid tert-butyl ester III:
[0042] Under nitrogen protection, 46g of zinc powder was added to 400mL of tetrahydrofuran, stirred for 20min, then 34.5g of (S)-3-hydroxy-4-chloro-butyronitrile was added, and then 56.2g of tert-butyl bromoacetate was slowly added dropwise at room temperature. After the temperature was raised to reflux for 3 hours, 2 mol / L hydrochloric acid was slowly added dropwise to adjust the pH to 5-6, and the reaction was stirred at room temperature for 2 hours. Add 200mL of ethyl acetate and 200mL of water for extraction, extract the aqueous layer with 100mL of ethyl acetate, combine the organic phases, wash once with 100mL of water, dry the organic phase over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain 50.6g of oily substance III.
Embodiment 2
[0043] The preparation of embodiment 2 (S)-6-chloro-5-hydroxyl-3-oxohexanoic acid tert-butyl ester III:
[0044] Under nitrogen protection, add 69.0g of zinc powder to 400mL of dimethoxyethane, stir for 20min, then add 69g of (S)-3-hydroxy-4-chloro-butyronitrile, then slowly add 112.4g of bromoacetic acid dropwise at room temperature For tert-butyl ester, heat up to reflux for 3.5 hours, slowly add 2 mol / L hydrochloric acid dropwise, adjust the pH to 5-6, and stir at room temperature for 2.5 hours. Add 450mL of ethyl acetate and 450mL of water for extraction, extract the aqueous layer with 200mL of ethyl acetate, combine the organic phases, wash once with 150mL of water, dry the organic phase over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain 97.5g of oily substance III.
Embodiment 3
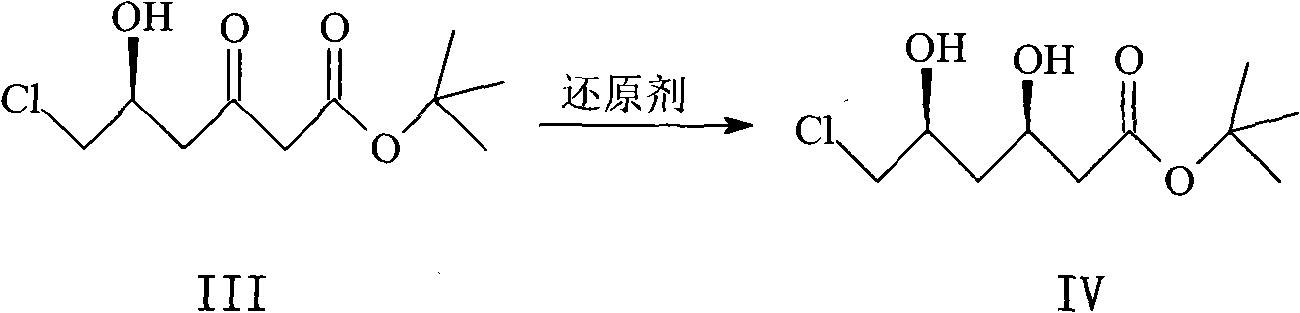
[0045] The preparation of embodiment 3 (R, S)-6-chloro-3,5-dihydroxyhexanoic acid tert-butyl ester IV
[0046] Dissolve 93.8g of compound III in 1.25L of dry tetrahydrofuran and 600mL of methanol, cool to -80°C under the protection of nitrogen, add 427mL of diethylmethoxyborane (1mol / L solution in tetrahydrofuran), stir the reaction for 20min, then add Sodium borohydride 16.5g, react at this temperature for 3h, add 200mL acetone and 80mL30% hydrogen peroxide, react at -50°C for 30min, pour the reaction system into 800mL water, extract with ethyl acetate (400mL×3), combine the organic phase , the organic phase was washed with water (100 mL×3), dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain solid IV, which was recrystallized from n-hexane to obtain 74.6 g of a pale yellow solid, with a yield of 79.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com