Rosuvastatin calcium tablet and preparation method thereof
A technology for rosuvastatin calcium and tablets, which is applied in the field of preparation of rosuvastatin calcium tablets and rosuvastatin calcium tablets, can solve the problems of uncontrollable quality stability, low bioavailability and dissolution rate. The problem is not high, to achieve the effect of controllable product quality, high quality stability and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
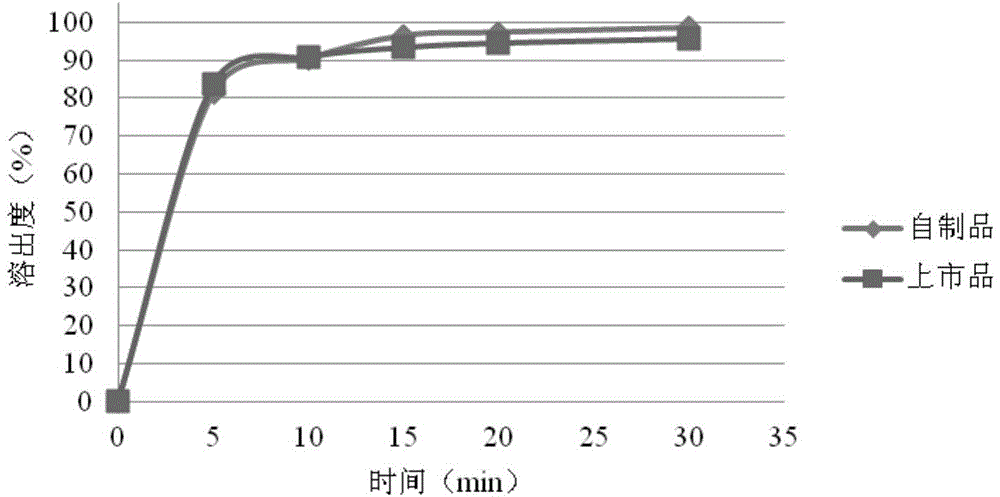
[0050] The rosuvastatin calcium tablet of the present embodiment comprises the following components by weight: rosuvastatin calcium 5.21g, microcrystalline cellulose 20.08g, lactose 78.71g, calcium carbonate 40g, cross-linked polyvinylpyrrolidone 4.5g, Magnesium stearate 1.5g. The weight of the rosuvastatin tablet in this embodiment is 150 mg, and the specification of rosuvastatin is 5 mg, and a total of 1000 tablets are made.
[0051] The preparation method of the rosuvastatin calcium tablet of the present embodiment comprises the following steps:
[0052] 1) Rosuvastatin calcium and cross-linked polyvinylpyrrolidone are respectively pulverized and passed through a 100-mesh drug sieve for subsequent use; microcrystalline cellulose, lactose, calcium carbonate, and magnesium stearate are respectively passed through a 100-mesh drug sieve for subsequent use;
[0053] 2) Take the prescribed amount of rosuvastatin calcium and lactose and place them in a high-efficiency wet mixing ...
Embodiment 2
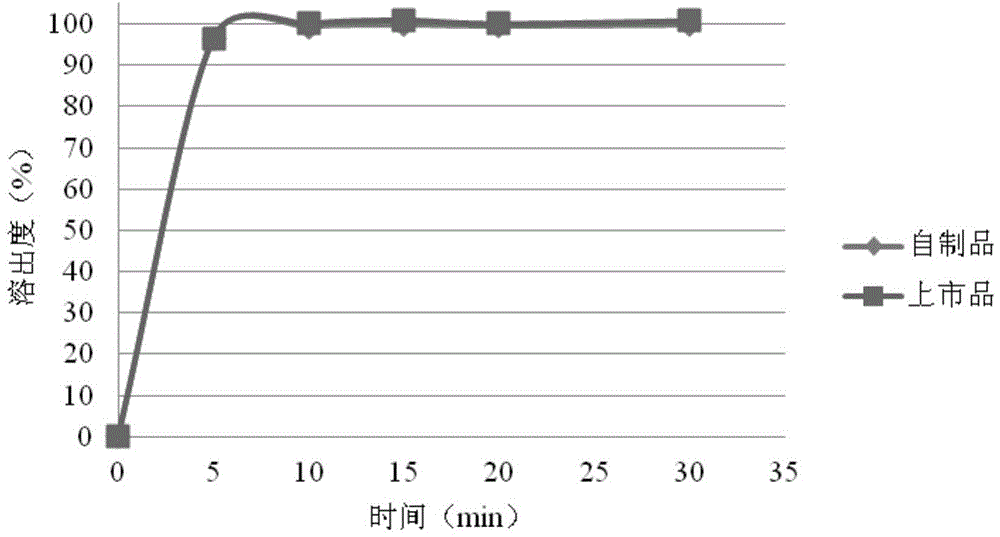
[0057] The rosuvastatin calcium tablet of the present embodiment comprises a tablet core and a coating, and the tablet core comprises the following components by weight: rosuvastatin calcium 5.21g, microcrystalline cellulose 20.08g, lactose 78.71g, carbonate 40g of calcium, 4.5g of cross-linked polyvinylpyrrolidone, 1.5g of magnesium stearate; the weight of the tablet chip is 150mg, and the rosuvastatin is counted as 5mg specification, and 1000 tablets are made in total;
[0058] The coating is mainly made of the following raw materials: Opadry 295K620010Yellow; the mass of the coating is 4% of the mass of the tablet core.
[0059] The preparation method of the rosuvastatin calcium tablet of the present embodiment comprises the following steps:
[0060] 1) Rosuvastatin calcium and cross-linked polyvinylpyrrolidone are respectively pulverized and passed through a 100-mesh drug sieve for subsequent use; microcrystalline cellulose, lactose, calcium carbonate, and magnesium steara...
Embodiment 3
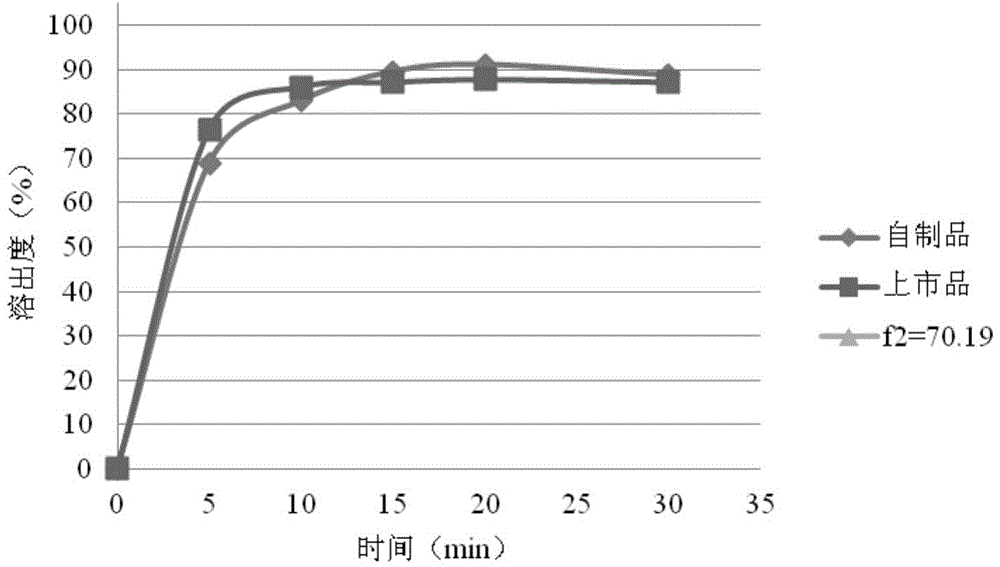
[0066] The rosuvastatin calcium tablet of the present embodiment comprises the following components by weight: rosuvastatin calcium 10.42g, microcrystalline cellulose 20.08g, lactose 73.5g, calcium carbonate 40g, cross-linked polyvinylpyrrolidone 4.5g, Magnesium stearate 1.5g. The weight of the rosuvastatin tablet in this embodiment is 150 mg, and the specification of rosuvastatin is 10 mg, and a total of 1000 tablets are made.
[0067] The preparation method of the rosuvastatin calcium tablet of the present embodiment comprises the following steps:
[0068] 1) Rosuvastatin calcium and cross-linked polyvinylpyrrolidone are respectively pulverized and passed through a 100-mesh drug sieve for subsequent use; microcrystalline cellulose, lactose, calcium carbonate, and magnesium stearate are respectively passed through a 100-mesh drug sieve for subsequent use;
[0069] 2) Take the prescribed amount of rosuvastatin calcium and lactose and place them in a high-efficiency wet mixing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com