Tablet containing Rosuvastatin calcium and preparation process thereof
A technology of rosuvastatin calcium and tablet, applied in the field of medicine, can solve problems such as complicated process and achieve the effects of easy promotion, small dissolution difference and strong compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Preparation of rosuvastatin calcium tablet
[0034] Rosuvastatin 5%
[0035] Lactose 52.9%
[0036] Hydroxypropyl Cyclodextrin 33%
[0037] Cross-linked polyvinylpyrrolidone 8%
[0039] Medicinal Iron Oxide 0.1%
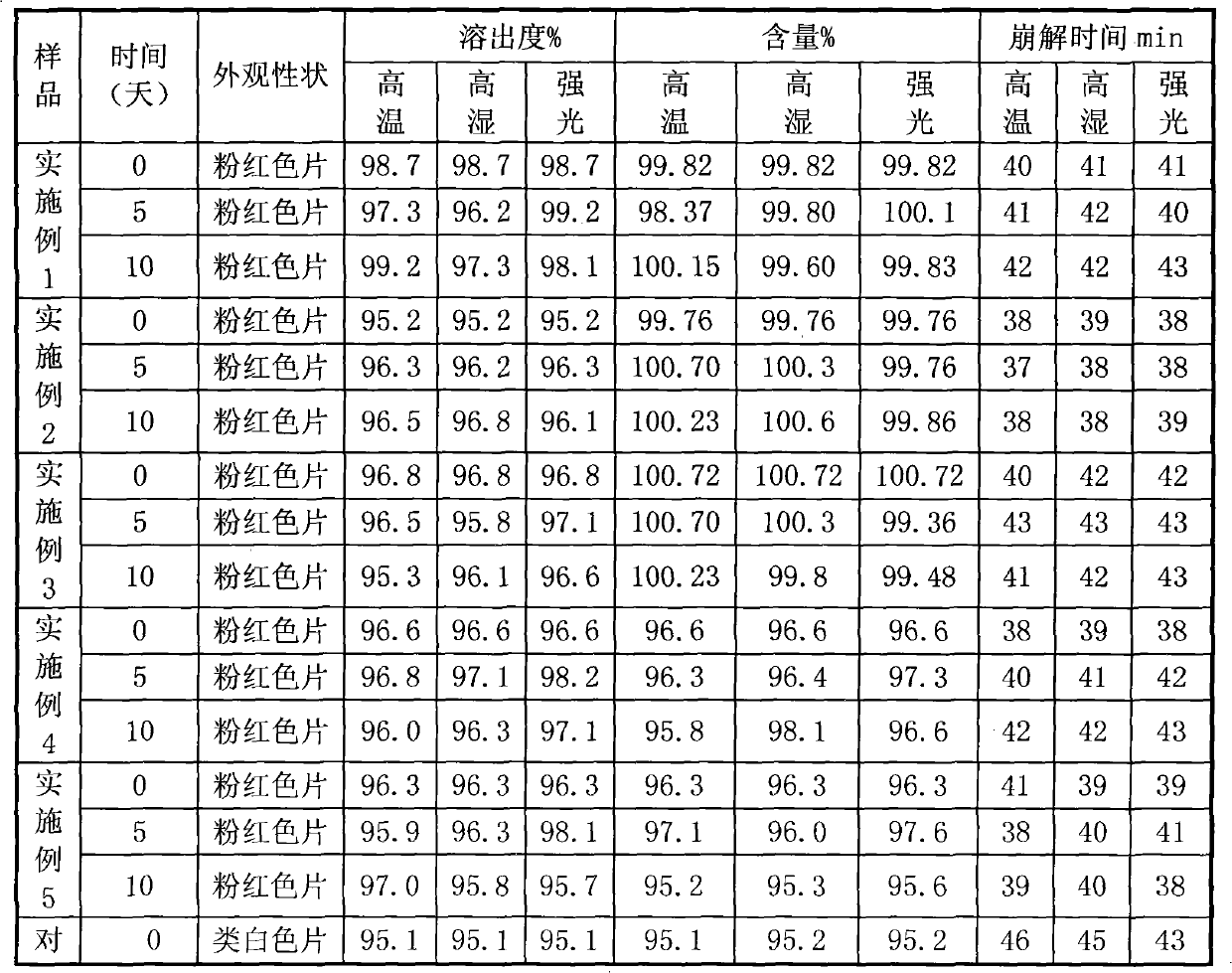
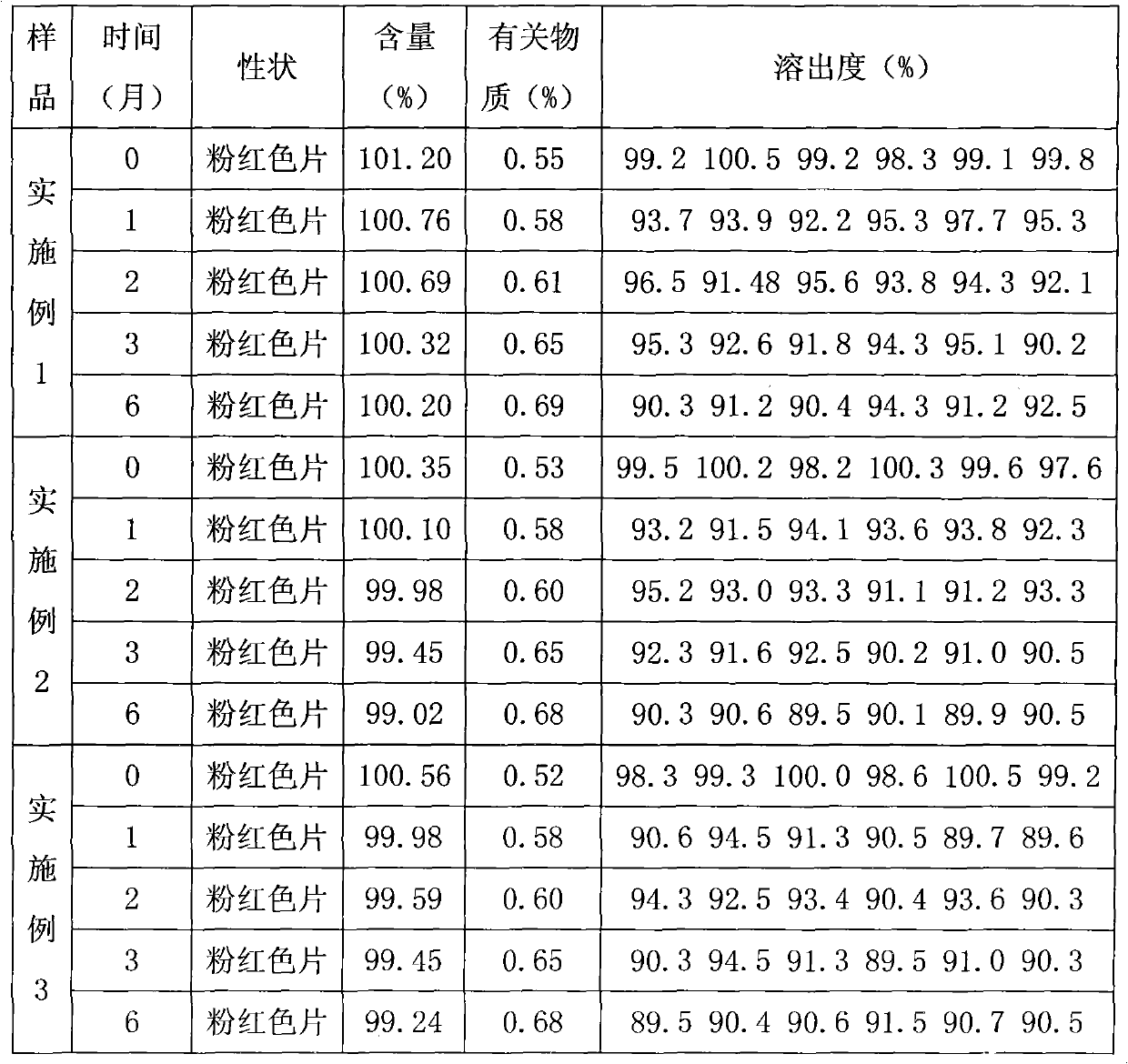
[0040] The specific preparation method is as follows: pass medicinal iron oxide red and raw materials through 80 mesh sieve respectively, weigh according to the prescription amount, mix evenly, then mix evenly with hydroxypropyl cyclodextrin according to the method of equal amount addition, pass through 60 mesh sieve, and then Mix evenly with the rest of the excipients in the prescribed amount, measure the content of the semi-finished product, calculate the weight of the tablet, and press the tablet. The appearance and hardness of the prepared tablet are very good, and the dissolution rate is 99.35% ± 0.8% in 45 minutes.
Embodiment 2
[0041] The preparation of embodiment 2 rosuvastatin calcium tablet
[0042] Rosuvastatin 10%
[0043] Lactose 50.9%
[0044] Hydroxypropyl Cyclodextrin 30%
[0045] Cross-linked polyvinylpyrrolidone 8%
[0047] Medicinal iron oxide red 0.1%
[0048] The specific preparation method is as follows: pass medicinal iron oxide red and raw materials through 80 mesh sieve respectively, weigh according to the prescription amount, mix evenly, then mix evenly with hydroxypropyl cyclodextrin according to the method of equal amount addition, pass through 60 mesh sieve, and then Mix evenly with the rest of the excipients in the prescribed amount, measure the content of the semi-finished product, calculate the weight of the tablet, and press the tablet. The appearance and hardness of the prepared tablet are very good, and the dissolution rate is 101.23% ± 0.5% in 45 minutes.
Embodiment 3
[0049] The preparation of embodiment 3 rosuvastatin calcium tablet
[0050] Rosuvastatin 5%
[0051] Lactose 44%
[0052] Hydroxypropyl Cyclodextrin 43.9%
[0053] Cross-linked polyvinylpyrrolidone 8%
[0055] Medicinal iron oxide red 0.1%
[0056] The specific preparation method is as follows: pass medicinal iron oxide red and raw materials through 80 mesh sieve respectively, weigh according to the prescription amount, mix evenly, then mix evenly with hydroxypropyl cyclodextrin according to the method of equal amount addition, pass through 60 mesh sieve, and then Mix evenly with the rest of the excipients in the prescribed amount, measure the content of the semi-finished product, calculate the weight of the tablet, and press the tablet. The appearance and hardness of the prepared tablet are very good, and the dissolution rate is 99.35% ± 0.8% in 45 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com