Preparation methods for rosuvastatin calcium and intermediates thereof
A technology of rosuvastatin and intermediates, applied in the field of drug synthesis, which can solve the problems of unsuitability for industrial application, transportation, storage, use and post-processing of n-butyllithium, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
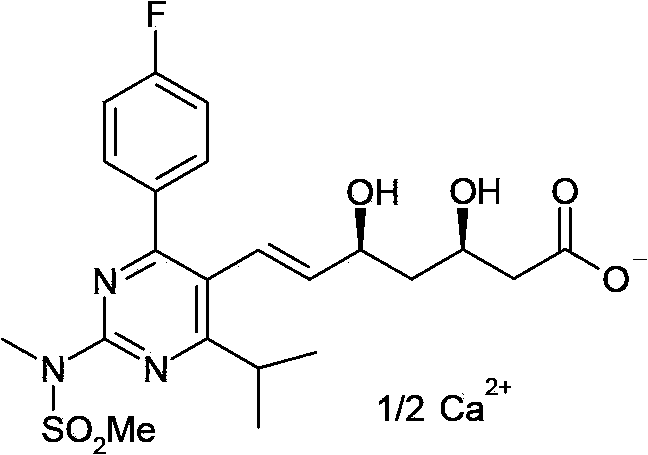
[0061] The present invention provides an intermediate compound for preparing rosuvastatin calcium shown in formula II, the structure of which is as follows:
[0062]
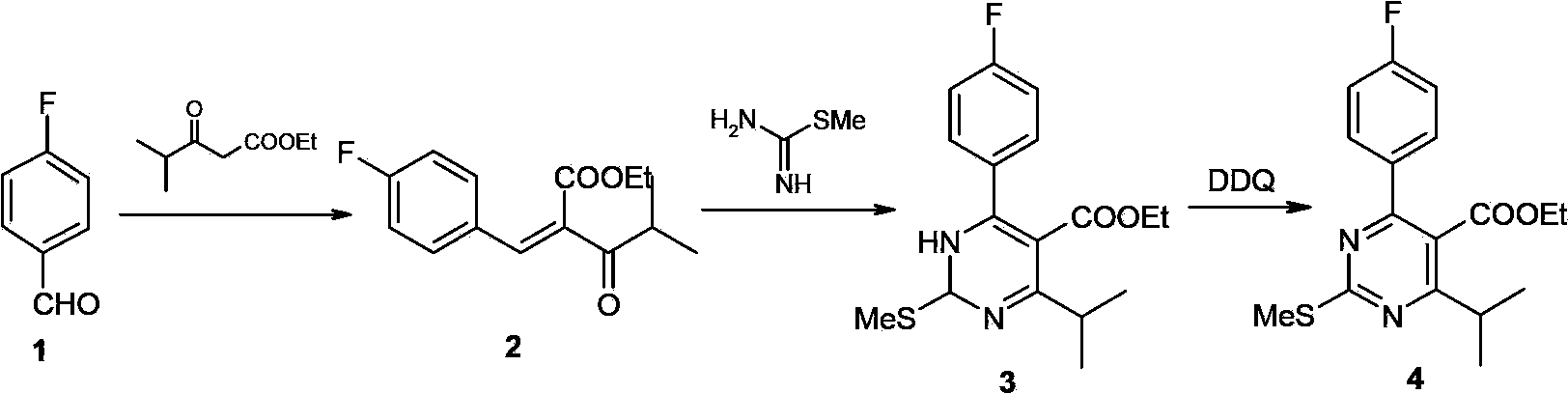
[0063] The compound of formula II used in the present invention can be prepared by conventional methods in the art, and can also be preferably prepared by the following steps: 1~4 In a halogenated hydrocarbon solvent (such as dichloromethane, chloroform, or a combination thereof), at a certain temperature (such as -5 ~ 30 ° C, preferably 0 ~ 25 ° C), the compound of formula I and form The sulfonyl chloride is reacted for a period of time (eg, 2-8 hours or 3-5 hours) to form a compound of formula II.
[0064]
[0065] The intermediate shown in formula III
[0066] The present invention provides an intermediate compound for preparing rosuvastatin calcium shown in formula III, the structure of which is as follows:
[0067]
[0068] The compound of formula III used in the present invention can be prepared...
Embodiment 1
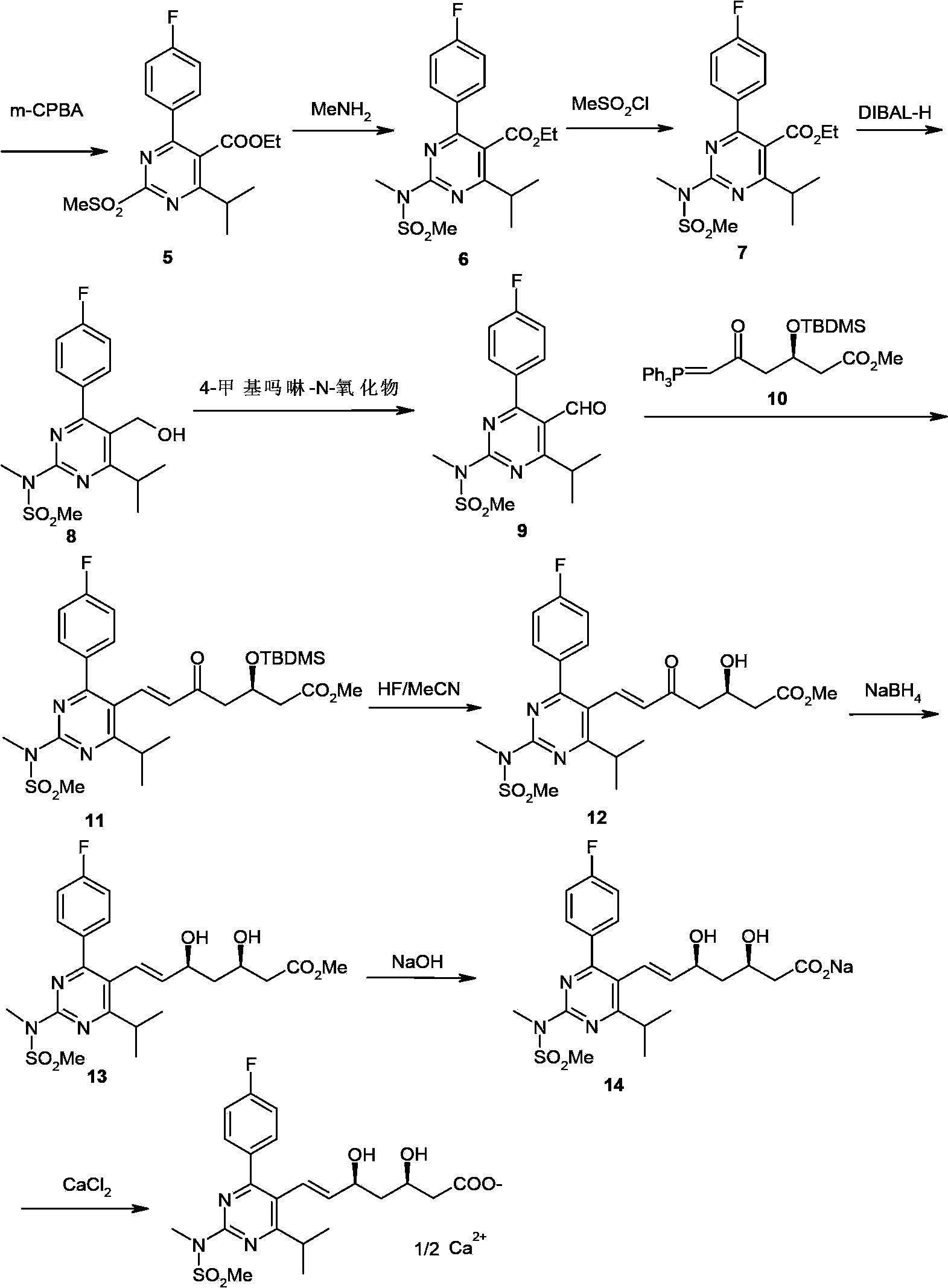
[0126] 1.1 Preparation of formula II compound
[0127] Add formula I compound (29.0g, 0.10mol), dichloromethane 300ml, triethylamine (12.2g, 0.12mol) into the reaction flask, cool down to 0°C, slowly add methanesulfonyl chloride (12.5g, 0.11mol) ). After the addition, it was raised to room temperature and reacted for 4 hours. After the reaction was complete, 300ml of water was added and stirred for 15 minutes. The layers were separated, and the organic layer was washed successively with saturated ammonium chloride solution and saturated brine, and anhydrous MgSO 4 Dry and concentrate to dryness under reduced pressure to obtain 35.0 g of white solid with a yield of 94.9%. MS (ESI) m / z: (M+H) = 369.4.
[0128] 1.2 Preparation of Formula III Compound
[0129] Add the compound of formula II (35.0g, 0.095mol) and 350ml of toluene into the reaction bottle, cool down to -10°C, slowly add 95ml of 2mol / L diisobutylaluminum hydride (DIBAl-H) toluene solution dropwise, and finish th...
Embodiment 2
[0141] 2.1 Preparation of formula II compound
[0142] Add the compound of formula I (15.0g, 0.052mol), 150ml of dichloromethane, and pyridine (4.7g, 0.059mol) into the reaction flask, cool down to 0°C, and slowly add methanesulfonyl chloride (8.6g, 0.073mol) dropwise. After the addition, it was raised to room temperature and reacted for 4 hours. After the reaction was complete, 300ml of water was added and stirred for 15 minutes. The layers were separated, and the organic layer was washed successively with saturated ammonium chloride solution and saturated brine, and anhydrous MgSO 4 Dry and concentrate to dryness under reduced pressure to obtain 18.4 g of white solid with a yield of 96.7%. MS (ESI) m / z: (M+H) = 369.4.
[0143] 2.2 Preparation of Formula III Compound
[0144] Add the compound of formula II (18.4g, 0.050mol) and 200ml of toluene into the reaction bottle, cool down to -10°C, slowly add 60ml of toluene solution of 2mol / L lithium aluminum hydride dropwise, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com