Process for preparing amorphous rosuvastatin calcium free of impurities
一种罗苏伐他汀钙、罗苏伐他汀的技术,应用在无定型罗苏伐他汀钙领域,能够解决产率和物理状态不可重复、依赖、变成等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
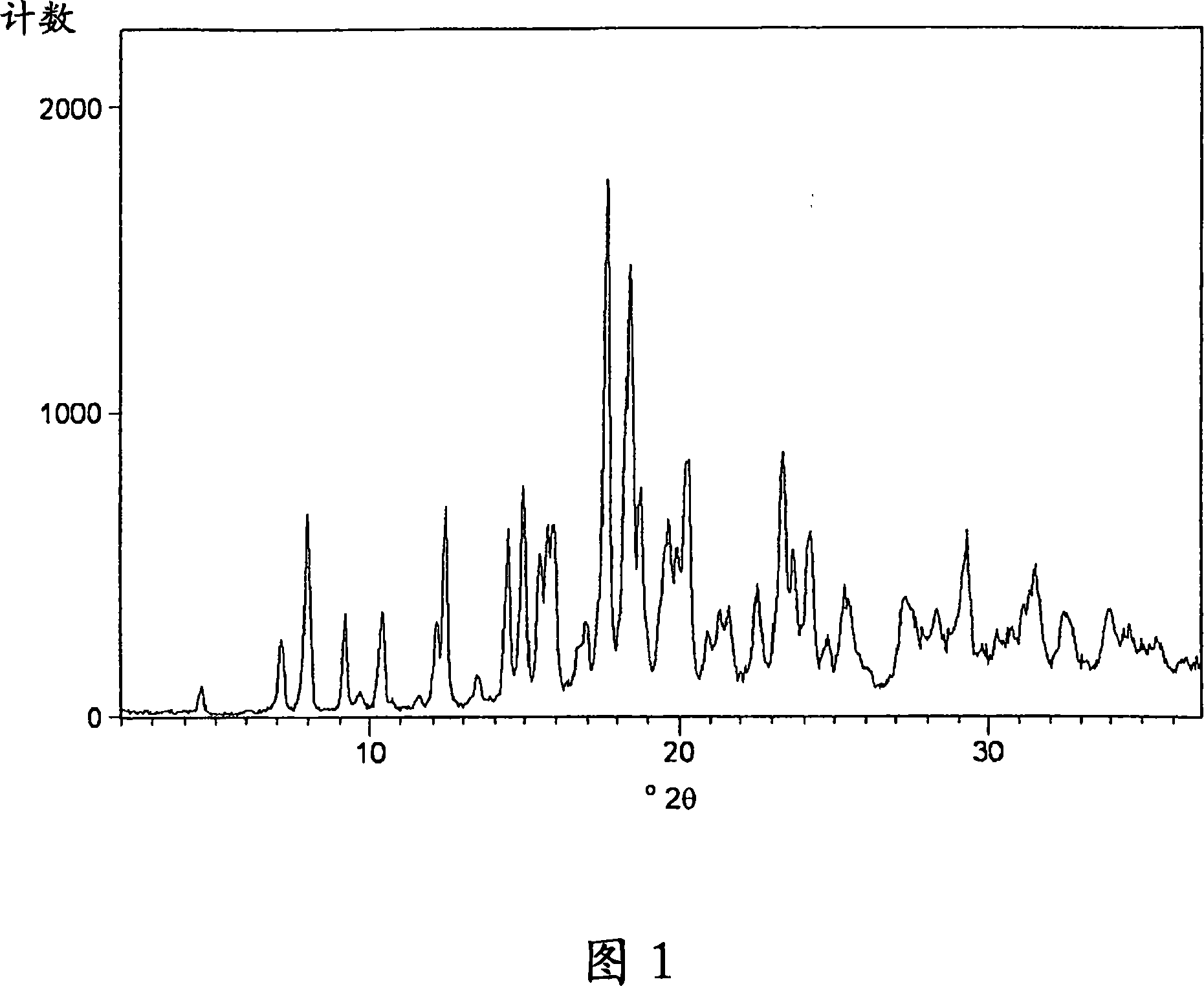
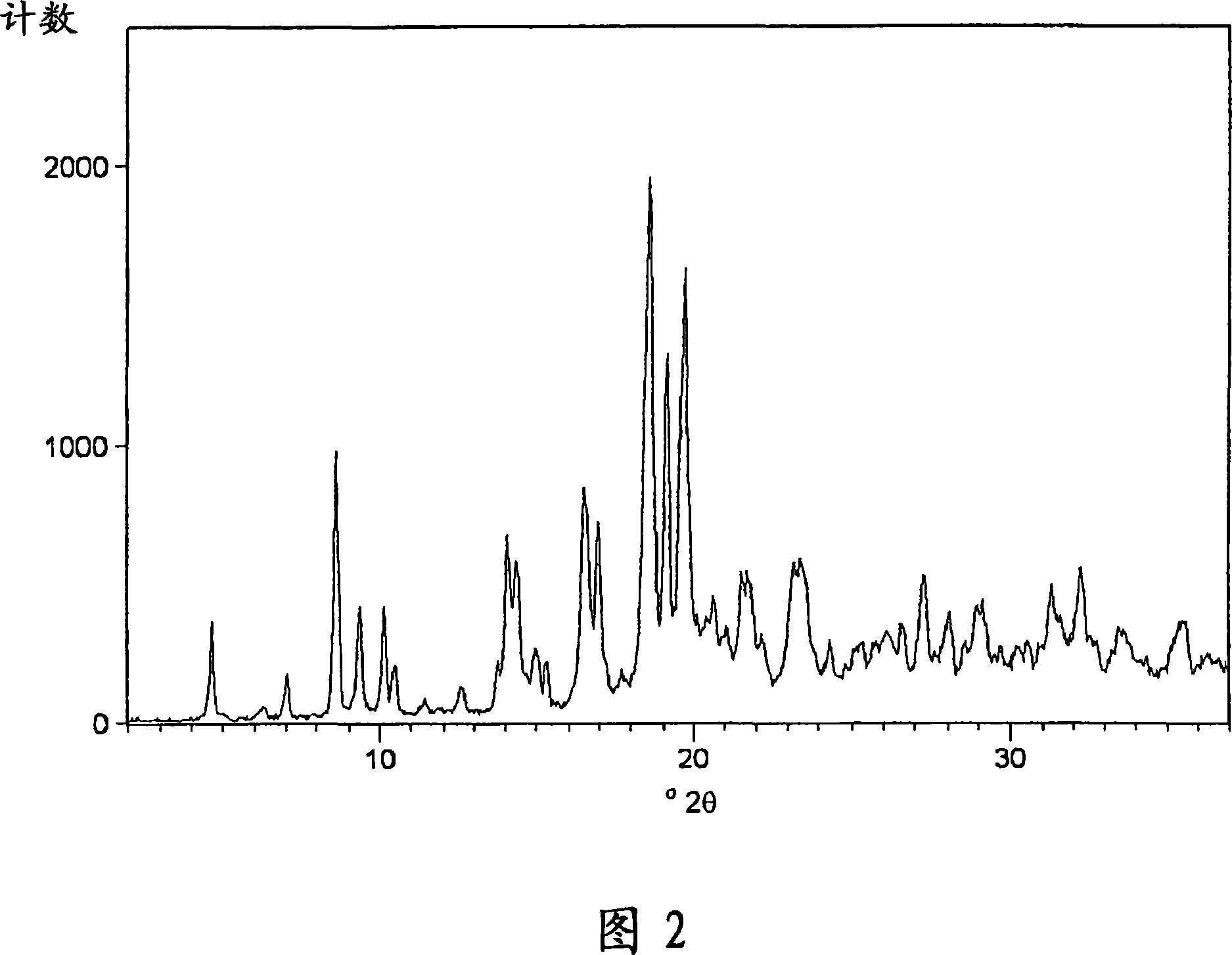
Image
Examples
no. 1 approach
[0047] According to a first embodiment of the present invention, rosuvastatin C 1 -C 5 Alkyl ester, more preferably rosuvastatin tert-alkyl ester, most preferably rosuvastatin tert-butyl ester or rosuvastatin lactone in an organic nitrogenous base optionally containing an organic aprotic solvent such as THF and water Cleavage in , wherein alkyl represents methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, pentyl or tert-amyl.
[0048] The used organic nitrogenous base of the inventive method is selected from:
[0049] a) Guanidines of the following formula:
[0050]
[0051] where R 1 , R 2 , R 3 , R 4 and R 5 Each independently represents a hydrogen atom, a straight chain or a branched chain C 1 -C 6 Alkyl or C 1 -C 6 Cycloalkyl, or R 1 , R 2 , R 3 , R 4 and R 5 Each pair in independently represents the C connected to form a ring 1 -C 6 alkylene;
[0052] b) amidines of the following formula:
[0053]
[0054] where R 1 , R 2 , R 3...
Embodiment 1
[0099] Hydrolysis of rosuvastatin tert-butyl ester in aqueous amine solution
[0100] 7.5g rosuvastatin tert-butyl ester
[0101] 38ml demineralized water
[0102] 2-5 equivalents of amine
[0103] The reaction was stirred with water as solvent in an autoclave at 98°C-100°C for 1-4 hours. The reaction mixture was sampled and analyzed by HPLC ("High Pressure Liquid Chromatography") to determine the completion of the reaction. The results are shown in Table 1.
[0104] Table 1
[0105] amine
Embodiment 2
[0107] Hydrolysis of Rosuvastatin Lactone in Aqueous N-Methylcyclohexylamine
[0108] 0.5g rosuvastatin lactone
[0109] 0.5ml N-methylcyclohexylamine
[0110] 3.0ml demineralized water
[0111] The reaction was stirred with solvent at 90°C for 1 hour to form a clear solution and analyzed as described in Example 1. HPLC analysis showed that the starting lactone was completely consumed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com