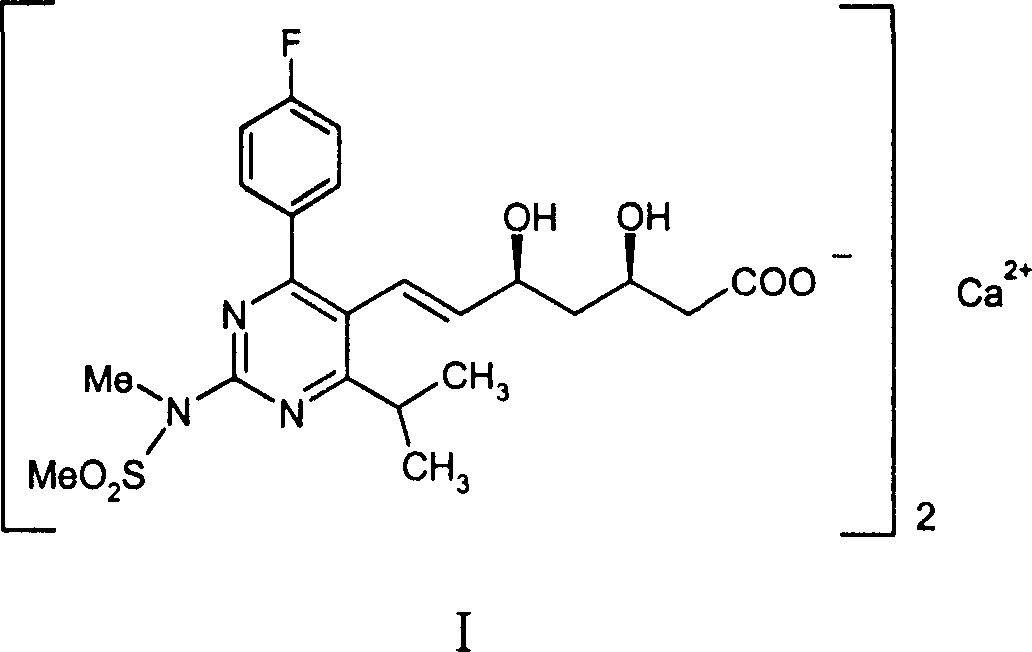
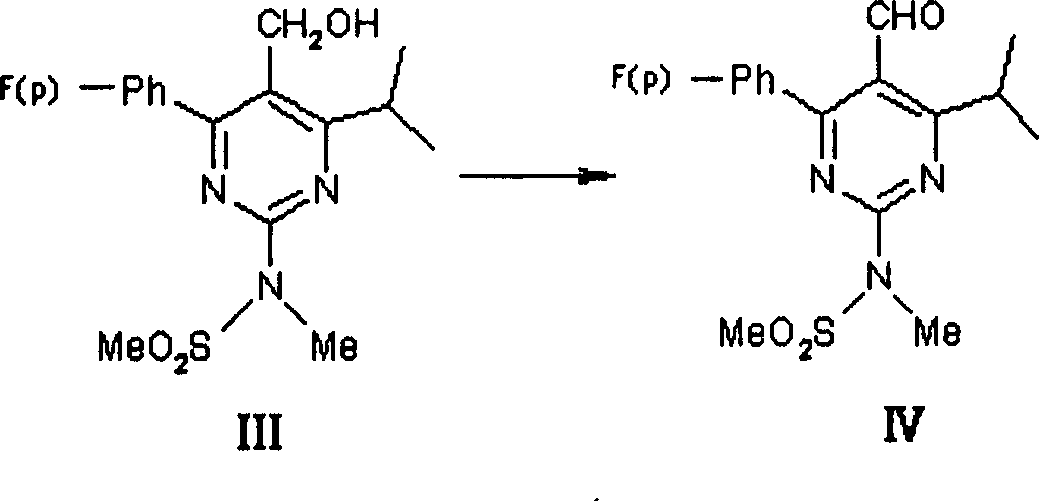
Method for preparing intermediate of synthesizing rosuvastatin calcium
A technology of rosuvastatin calcium and intermediates, which is applied in the field of intermediate preparation, can solve the problems of long reaction time, high temperature, increased process cost and the like, and achieves the effects of mild reaction conditions, short time and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
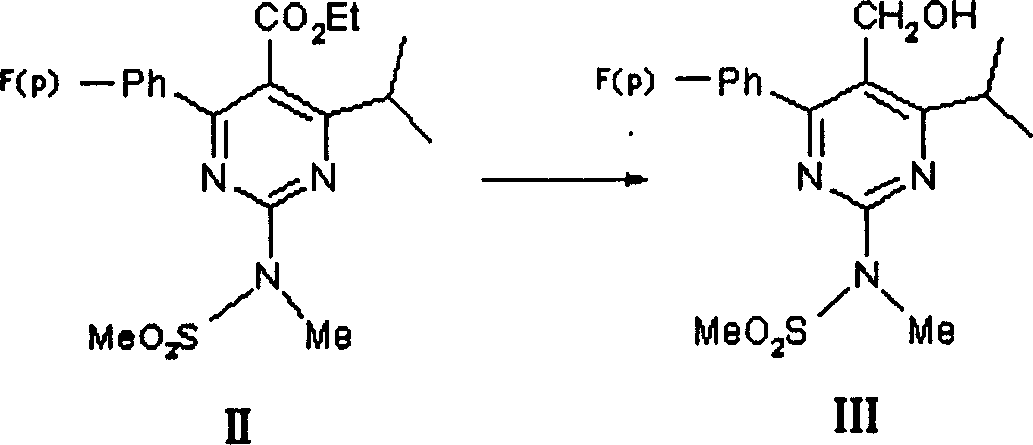
[0050] Preparation of [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyrimidin-5-yl]methanol (III)
[0051] Potassium borohydride (2.8g, 53.8mmol) was added to 1,4-dioxane (50ml), then dried anhydrous zinc chloride (3.6g, 26.5mmol), and stirred at 10°C for 2 hours. A solution of the starting ester (II) (5.0 g, 12.7 mmol) dissolved in 1,4-dioxane (30 ml) was added dropwise. Heat to 90°C for 2 hours, filter, concentrate the filtrate and dilute with ethyl acetate (50ml), adjust the pH value to 3 with 1N hydrochloric acid, separate the layers, extract the aqueous layer with ethyl acetate (20ml×2), combine the organic layer, washed with 10% sodium bicarbonate solution until neutral, washed with water, and concentrated to obtain 4.1 g of off-white solid III, with a yield of 91.8%.
Embodiment 2
[0053] Preparation of [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyrimidin-5-yl]methanol (III)
[0054] Lithium borohydride (1.2g, 54.5mmol) was added to 1,4-dioxane (50ml), and anhydrous aluminum chloride (3.5g, 26.2mmol) was added, and stirred at 35°C for 1.5 hours. A solution of the starting ester (II) (5.0 g, 12.7 mmol) dissolved in 1,4-dioxane (30 ml) was added dropwise. Heat to 80°C to react for 2 hours, filter, concentrate the filtrate and dilute with ethyl acetate, adjust the pH value to 3 with dilute hydrochloric acid, separate the layers, extract the aqueous layer with ethyl acetate, combine the organic layers, and wash with 10% sodium bicarbonate solution Wash until neutral, wash with water, and concentrate to obtain 3.7 g of off-white solid III, with a yield of 82.8%.
Embodiment 3
[0056] Preparation of [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyrimidin-5-yl]methanol (III)
[0057] Potassium borohydride (2.8g, 53.8mmol) was added to diethylene glycol diethyl ether (50ml), then dried anhydrous zinc chloride (3.6g, 26.5mmol), and stirred at 25°C for 3 hours. A solution of starting ester (II) (5.0 g, 12.7 mmol) dissolved in diethyl ether (30 ml) was added dropwise. Heat to 150°C to react for 1 hour, filter, concentrate the filtrate and dilute with ethyl acetate, adjust the pH value to 3 with dilute hydrochloric acid, separate the layers, extract the aqueous layer with ethyl acetate, combine the organic layers, and wash with 10% sodium bicarbonate solution Wash until neutral, wash with water, concentrate and purify by column chromatography to obtain 2.4 g of a white solid III with a yield of 53.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com