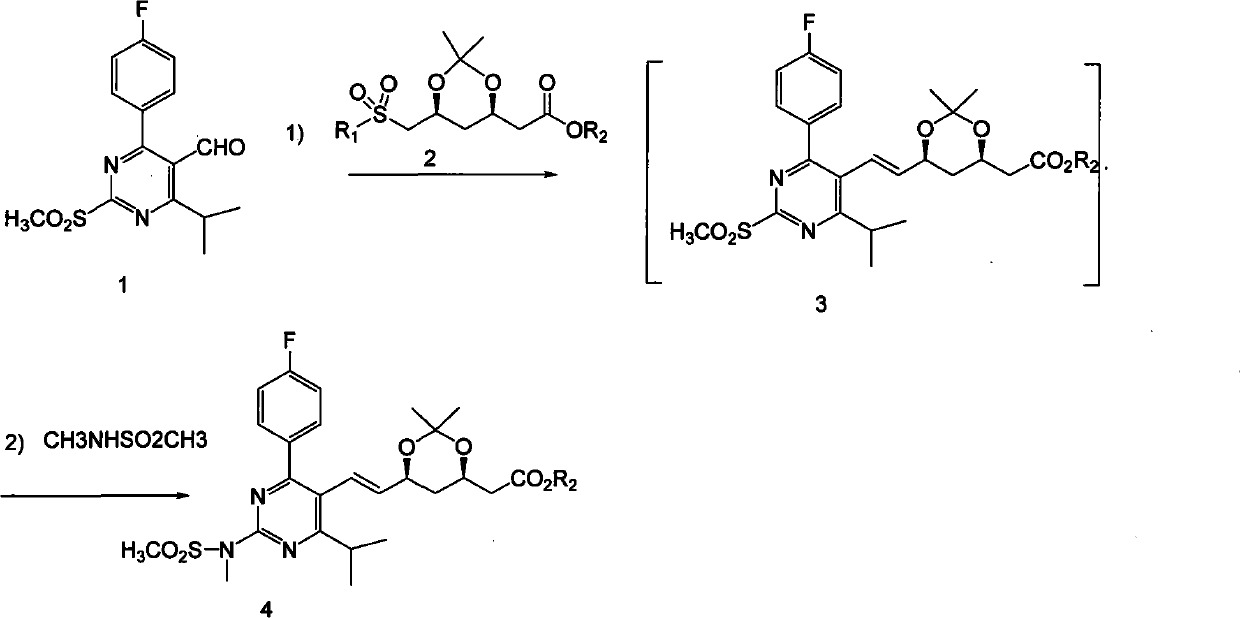
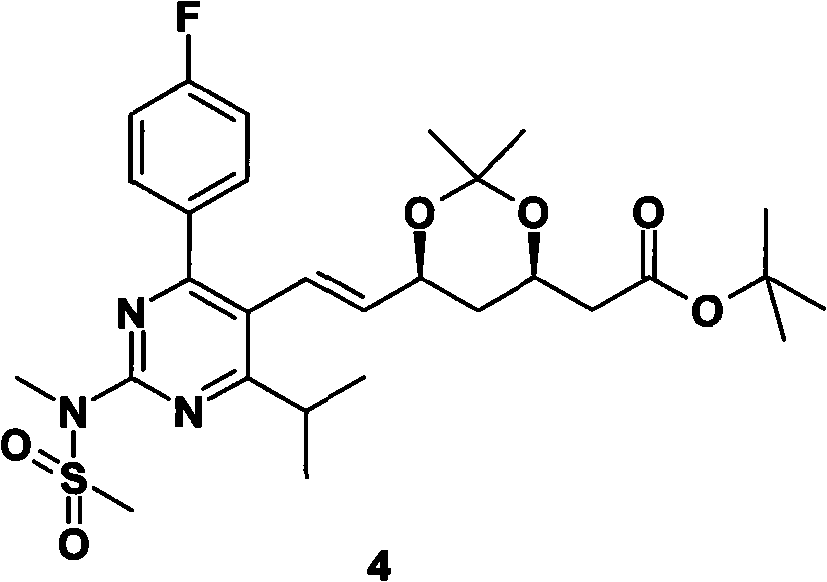
Method for preparing (3R, 5S, E)-7-{2-(N-methylsulphonylamino) -4-(4-fluorophenyl)-6-isopropyl-pyrimidine-5-yl}-2,2-dimethyl-3,5-dioxane-6-heptenoic acid
A technology of methylmethanesulfonamide group and methylmethanesulfonamide is applied in the field of pharmaceutical preparation, can solve the problems of complicated operation, high cost, unstable yield and the like, and achieves the effects of easy industrialization, simple operation and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 1000mL three-neck flask, add 15.0g of compound 1, 22.1 (1.05eq)g of compound 2, under argon protection, add 300mL of anhydrous tetrahydrofuran, cool to -60°C in a dry ice acetone bath, and drop 56mL of 1M hexamethyl The tetrahydrofuran solution of lithium disilazide was added, and the temperature was raised to room temperature 25° C. within 2 hours. Under the protection of argon, 4.1 g of 60% sodium hydrogen was added, and 7.62 g of N-methylmethanesulfonamide was added, and the reaction was carried out overnight. Under cooling, add 100mL ethyl acetate, 20mL saturated aqueous ammonium chloride solution, 20mL water, separate the liquids, extract the water phase with 200mL ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, 30°C The solvent was removed by rotary evaporation in a water bath to obtain the product (3R, 5S, E)-7-(2-(N-methylmethylsulfonamido)-4-(4-fluorophenyl)-6-isopropyl-pyrimidine- 5-yl)-2,2-...
Embodiment 2
[0024] In a 1000mL three-neck flask, add 15.0g of compound 1, 22.1g of compound 2, under argon protection, add 300mL of anhydrous tetrahydrofuran, cool to -60°C in a dry ice acetone bath, and add 70mL of 1M hexamethyldisilazylamine dropwise at this temperature Lithium base, after addition, rise to room temperature 25°C within 2 hours, under the protection of argon, add 3.73 grams of 60% sodium hydrogen and 10.16 grams of N-methylmethanesulfonamide, react overnight at 20-30°C, under cooling, add 100mL ethyl acetate, 20mL saturated aqueous ammonium chloride solution, 20mL water, separate the liquids, extract the aqueous phase with 200mL ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and remove by rotary evaporation in a water bath at 30°C. solvent to give the product (3R,5S,E)-7-(2-(N-methylmethylsulfonamido)-4-(4-fluorophenyl)-6-isopropyl-pyrimidin-5-yl) - tert-butyl 2,2-dimethyl-3,5-dioxane-6-heptenoate (18.95 g...
Embodiment 3
[0026] In a 1000mL three-neck flask, add 15.0g of compound 1, 22.1g of compound 2, under argon protection, add 300mL of anhydrous tetrahydrofuran, cool to -60°C in a dry ice acetone bath, add 6.52g of 60% sodium hydrogen in batches at this temperature After the addition is completed, the temperature rises to 20-30°C within 2 hours, and 10.16 g of N-methylmethanesulfonamide is added to react overnight at 20-30°C. Under cooling, add 100 mL of ethyl acetate, 20 mL of saturated ammonium chloride aqueous solution, and 20 mL of water , liquid separation, the aqueous phase was extracted with 200mL ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation in a water bath at 30°C to obtain the product (3R, 5S, E)-7-( 2-(N-Methylmethanesulfonamido)-4-(4-fluorophenyl)-6-isopropyl-pyrimidin-5-yl)-2,2-dimethyl-3,5-dioxo tert-butyl hexacyclo-6-heptenoate (18.31 g, yield: 68.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com