Method for determining content of rosuvastatin calcium and related substances thereof by employing HPLC (high performance liquid chromatography) method
A technology for rosuvastatin calcium and related substances, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problems of the gradient elution method, such as complicated isocratic elution, difficulty in popularization, high requirements on instruments, equipment and chromatographic columns, and the like, Achieving the effect of good separation, saving detection cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
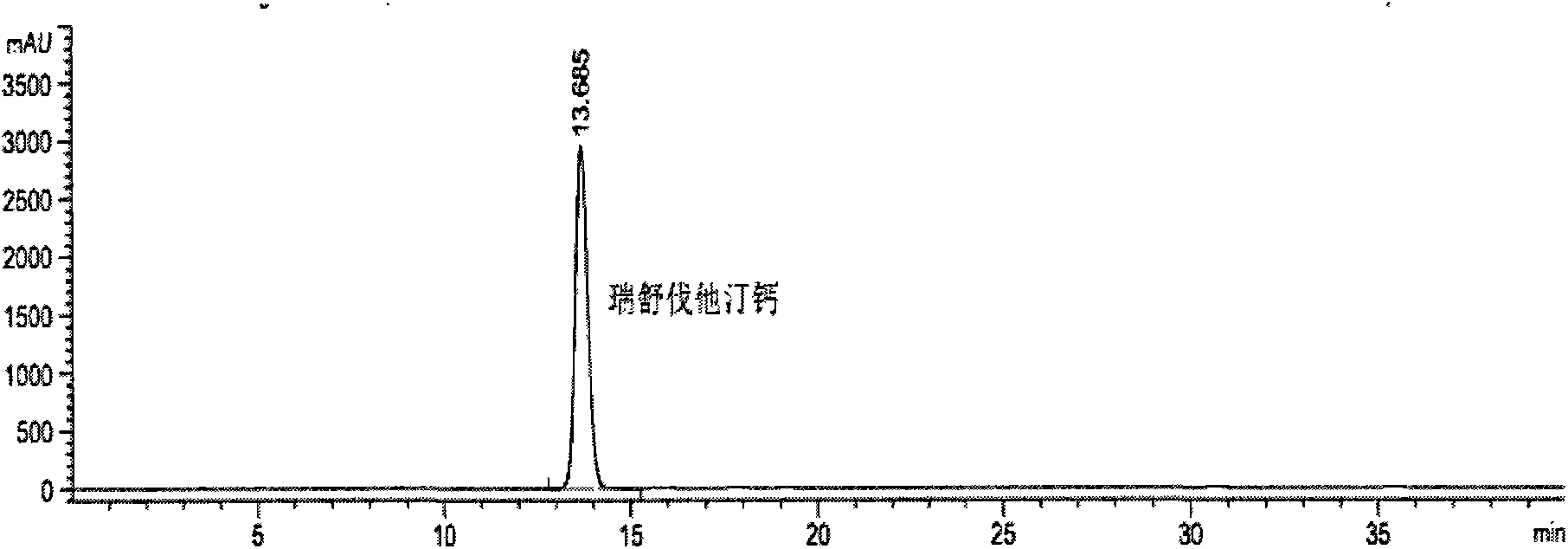
[0049] ① Rosuvastatin Calcium Reference Substance Solution: Accurately weigh 25 mg of Rosuvastatin Calcium Reference Substance, put it in a 50ml volumetric flask, add 30ml of water, dissolve it by ultrasonic, add water to dilute to the mark, shake well, and get it;
[0050] ② Preparation of degradation products:
[0051] Photodegradation product stock solution: Weigh 25 mg of rosuvastatin calcium reference substance, put it in a 50ml volumetric flask, add 30ml of water, dissolve it by ultrasonic, dilute to the mark with water, shake well; measure 10ml of this solution, put it in a clean glass vial, Put the vial under a light device (light intensity is about 15000-25000 lx·hour) at 25°C for 2 hours, and adjust the light time if necessary to obtain an appropriate amount of photodegradation products PDP1 and PDP2. Transfer the illuminated solution to a 25ml volumetric flask, add 5ml of acetonitrile to shake, add water to dilute to the mark, and shake well.
[0052]Lactonization ...
Embodiment 2
[0077] Preparation of the testing solution: get rosuvastatin calcium (sample 2), and prepare according to the preparation method of the testing solution in 1. Chromatographic conditions
[0078] High performance liquid chromatography: Agilent
[0079] Chromatographic column: Agilent C18 (150mm×4.6mm, 5μm)
[0080] Mobile phase: methanol: water (containing 0.8% trifluoroacetic acid) = 55:45
[0081] Flow rate: 0.75mL / min
[0082] Detection wavelength: 242nm
[0083] Injection volume: 10μL
[0084] Column temperature: 40°C
[0085] Other conditions are with embodiment 1.
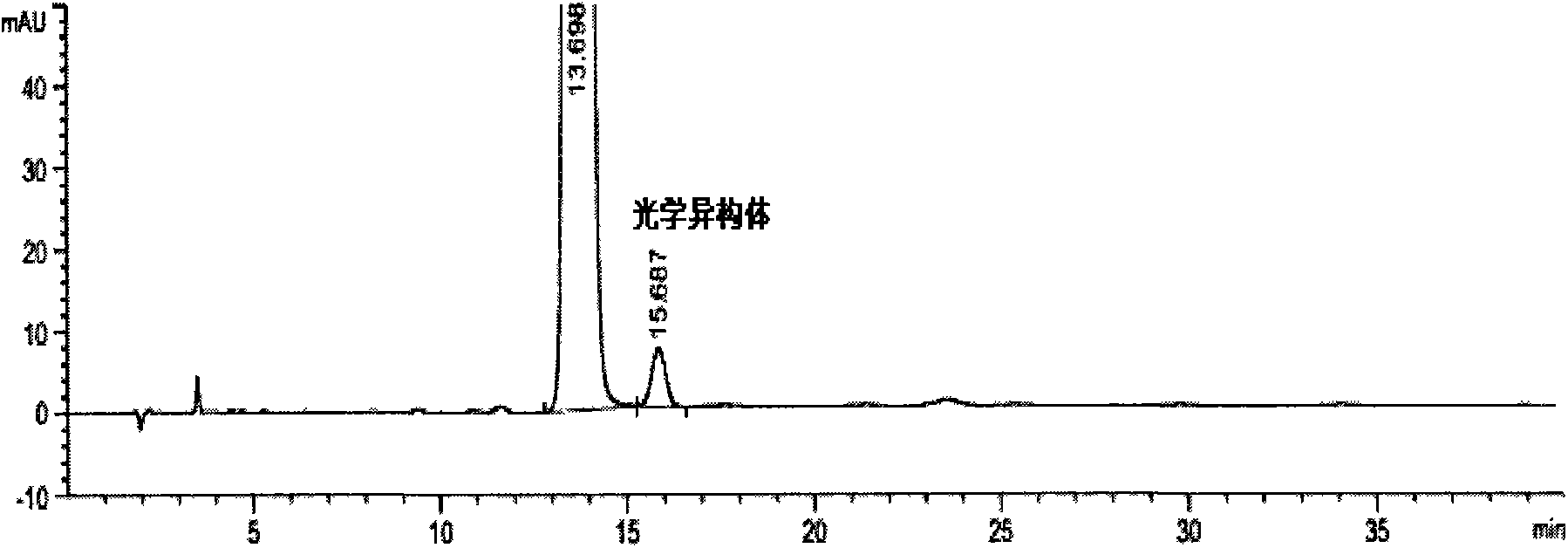
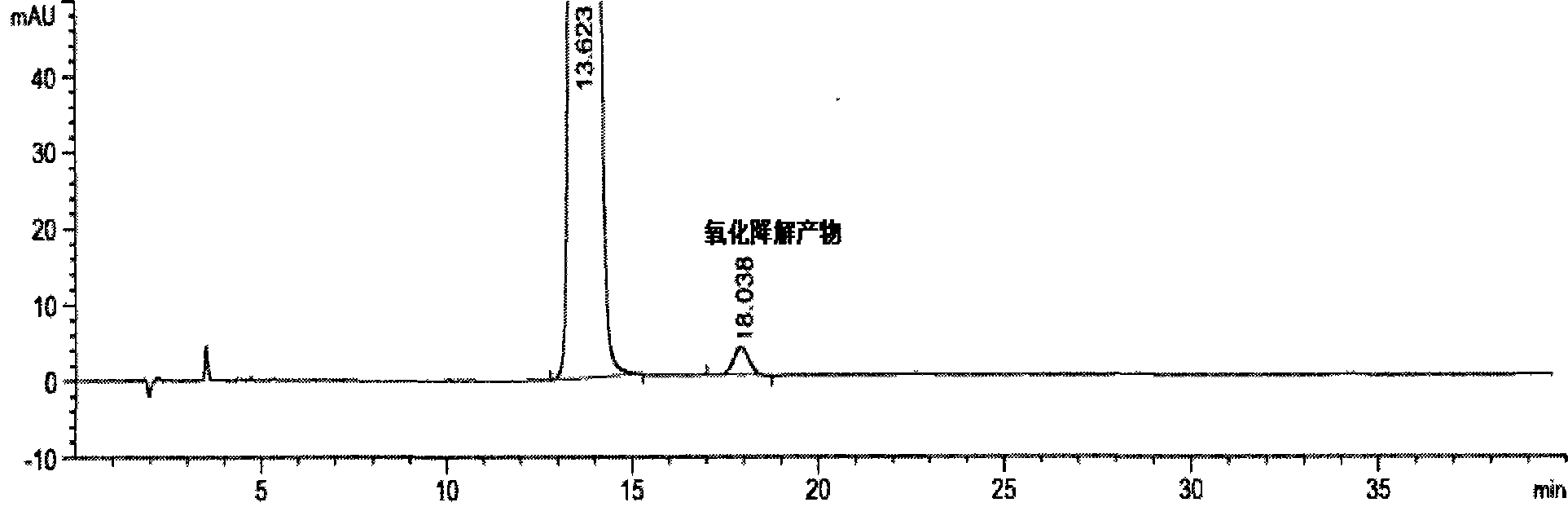
[0086] see results Figure 4 : Peaks 1-6 in the figure are the main peak of rosuvastatin calcium, the optical isomer peak of rosuvastatin calcium, and the oxidative degradation product ZD4522B 2 , Lactonization degradation product ZD4522 (3R, 5S). Under the above conditions, the peaks of rosuvastatin calcium and each product reached baseline separation, and the separation degrees from left to right we...
Embodiment 3
[0088] Preparation of the testing solution: get rosuvastatin calcium (sample 3), and prepare according to the preparation method of the testing solution in 1. Chromatographic conditions
[0089] High performance liquid chromatography: Agilent
[0090] Chromatographic column: Agilent C18 (150mm×4.6mm, 5μm)
[0091] Mobile phase: methanol: water (containing 0.1% trifluoroacetic acid) = 55:45
[0092] Flow rate: 0.75mL / min
[0093] Detection wavelength: 240nm
[0094] Injection volume: 10μL
[0095] Column temperature: 40°C
[0096] Other conditions are with embodiment 1.
[0097] see results Figure 5 : Peaks 1-6 in the figure are the main peak of rosuvastatin calcium and the oxidative degradation product ZD4522B 2 , lactonization degradation product ZD4522 (3R, 5S), photodegradation product ZD4522PDP1 and photodegradation product ZD4522PDP2. Under the above conditions, the peaks of rosuvastatin calcium and each product reached baseline separation, and the separation de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com