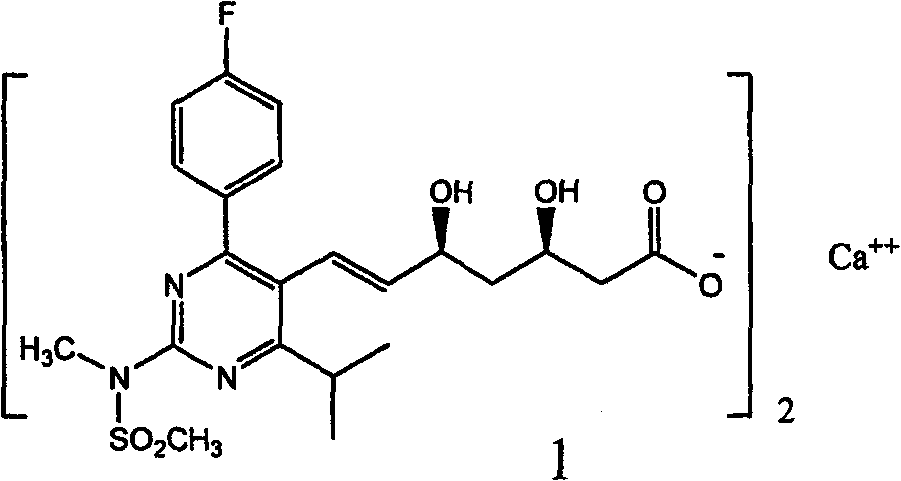
Rosuvastatin calcium tablet and preparation method
A technology of rosuvastatin calcium and tablet cores, which is applied in the field of rosuvastatin calcium tablets and its preparation, can solve the problem that the pharmaceutical composition cannot meet the storage period requirements, the stability of rosuvastatin calcium tablets decreases, and the preparation Problems such as product operation difficulties, to achieve the effect of good fluidity and compressibility, good stability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Rosuvastatin Calcium Tablets
[0034] Prescription composition (1000 tablets)
[0035]
[0036] Remarks: Lactose 90 average particle size 160μm; microcrystalline cellulose 302 average particle size 100μm.
[0037]Preparation method: first pass the rosuvastatin calcium raw material through a 100-mesh sieve, crospovidone, lactose, microcrystalline cellulose, and magnesium stearate respectively pass through a 60-mesh sieve, and then pass the prescribed amount of rosuvastatin calcium, Crospovidone, lactose, and microcrystalline cellulose are mixed evenly, and then magnesium stearate is added in the prescribed amount, mixed evenly, compressed into tablets, and film-coated.
[0038] Results: The tableting process was smooth, the tablet surface was smooth, and the difference in tablet weight was <±1.5%; the tablet surface was smooth and complete after coating. The dissolution rate of samples and related substances are as follows:
[0039]
[0040] Note:...
Embodiment 2
[0041] Embodiment 2: Rosuvastatin calcium tablet (screening to lactose)
[0042] With reference to embodiment 1 prescription composition and preparation method, just lactose 90 is replaced with 100 (average particle size 150μm), 70 (average particle size 212μm), Tablettose 80 (average particle size 180μm) or 100 (average particle size 150 μm), the experimental results are as follows:
[0043]
[0044] It can be seen that different types of lactose have little effect on drug dissolution rate and related substances, but slightly affect the difference in tablet weight. The difference in tablet weight obtained by using spray-dried lactose is small, and the difference in tablet weight obtained by using granular lactose is relatively large; The difference in tablet weight obtained by lactose with a larger diameter is small, but the difference in tablet weight obtained by all types of lactose is <±3.5%.
Embodiment 3
[0045] Embodiment 3: Rosuvastatin calcium tablet (screening to microcrystalline cellulose)
[0046] With reference to embodiment 1 prescription composition and preparation method, just microcrystalline cellulose 302 is replaced with PH 301 (average particle size 50μm), PH 102 (average particle size 90μm), 90M (average particle size 100μm), LP200 (average particle size 190μm), 301 (average particle size 65μm) and 200 (average particle size 250 μm), the experimental results are as follows:
[0047]
[0048] It can be seen that different types of lactose have little effect on drug dissolution rate and related substances, but slightly affect the difference in tablet weight. Within the average particle size range of 90-250 μm, the difference in tablet weight of the obtained rosuvastatin calcium tablets is <±1.5 %, but the differences in tablet weights obtained from the types of microcrystalline cellulose used were all <±3.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com