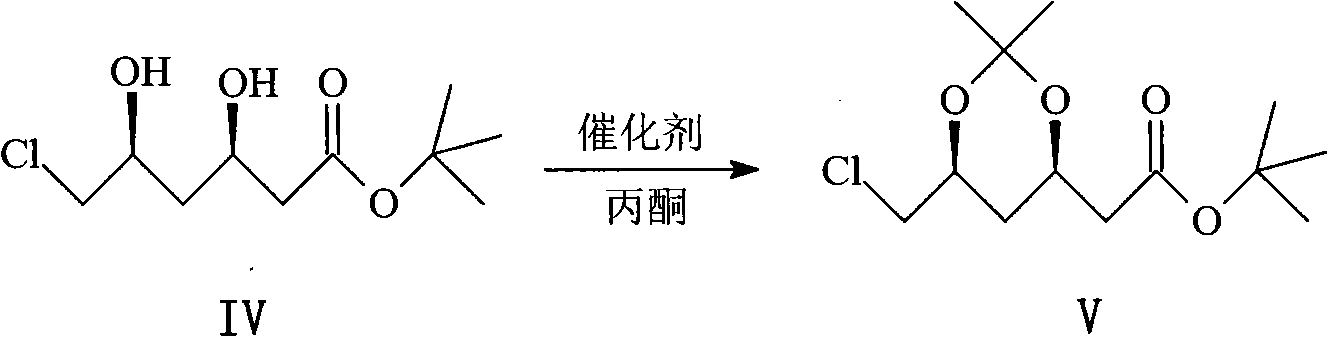
Preparation method of key intermediate of rosuvastatin calcium side chain
A technology of rosuvastatin calcium and intermediates, which is applied in the field of preparation of hypolipidemic drugs, can solve the problems of low yield of side chain and parent nucleus, long synthesis route, instability and the like, and achieves convenient industrial production and product yield. High, easy-to-use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
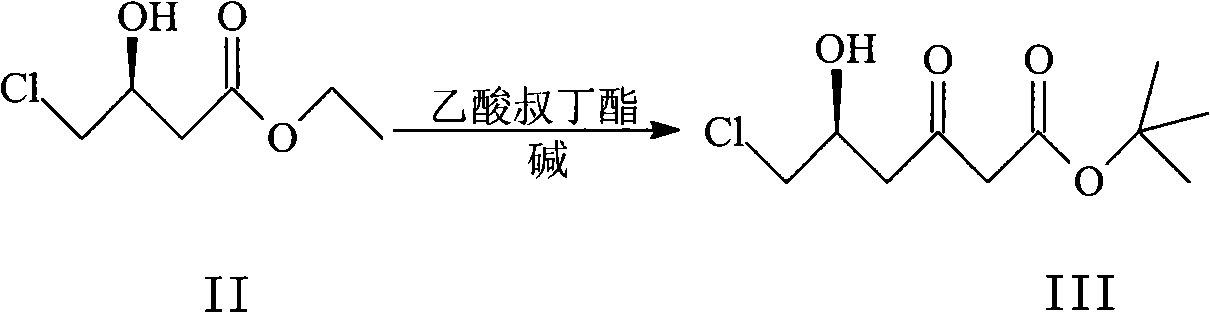
[0042] Embodiment 1 Preparation of (S)-6-chloro-5-hydroxyl-3-oxohexanoic acid tert-butyl ester III:
[0043] N 2Under protection, 225mL (1.61mol, 4.0eq) of diisopropylamine was dissolved in 1400mL of dried tetrahydrofuran, cooled to -78°C, and 64mL (4.0eq, 2.5mol / L) of tetrahydrofuran solution of n-butyllithium was slowly added dropwise. After reacting at this temperature for 30 min, 217.5 mL (1.61 mol, 4.0 eq) of tert-butyl acetate was slowly added dropwise, and then reacted at -78°C for 1 h. Slowly add 66.76g (402.2mmol, 1eq) II solution in 300mL tetrahydrofuran to the reaction system, the temperature is controlled at -78°C, after reacting at this temperature for 1h, the mixture is poured into 500mL10% hydrochloric acid, ethyl acetate (150mL×3 ), combined the organic phases, washed the organic phases with water (100 mL×2), dried over anhydrous sodium sulfate, evaporated the solvent under reduced pressure to obtain 93.8 g of oily matter.
Embodiment 2
[0044] Embodiment 2 Preparation of (S)-6-chloro-5-hydroxyl-3-oxohexanoic acid tert-butyl ester III:
[0045] N 2 Under protection, 168.8mL (1.21mol, 3.0eq) of diisopropylamine was dissolved in 1200mL of dried tetrahydrofuran, cooled to -65°C, and 483mL (3.0eq, 2.5mol / L), after reacting at this temperature for 30min, slowly add 163.1mL (1.21mol, 3.0eq) tert-butyl acetate dropwise, and then react at -55°C for 1h. Slowly add 66.76g (402.2mmol, 1eq) II solution in 300mL tetrahydrofuran to the reaction system, the temperature is controlled at -78°C, after reacting at this temperature for 1h, the mixture is poured into 500mL10% hydrochloric acid, ethyl acetate (150mL×3 ), combined the organic phases, washed the organic phases with water (100 mL×2), dried over anhydrous sodium sulfate, evaporated the solvent under reduced pressure to obtain 83.5 g of oily matter.
Embodiment 3
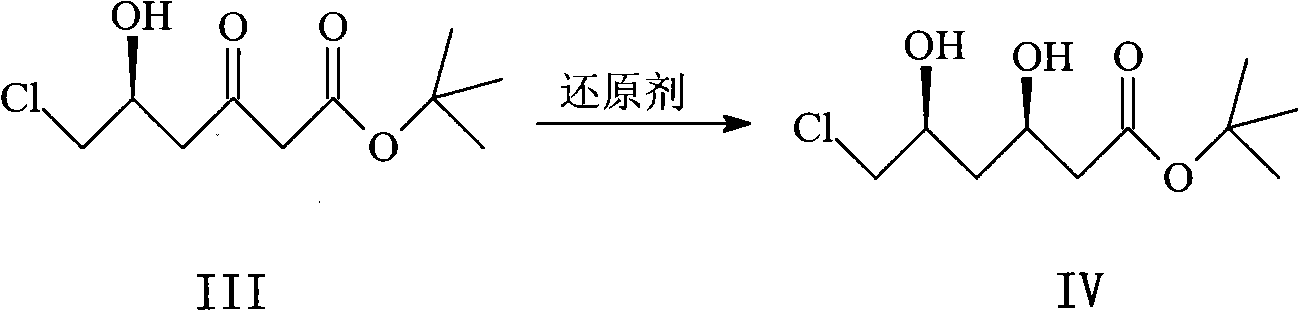
[0046] Example 3 Preparation of (R, S)-6-chloro-3,5-dihydroxyhexanoic acid tert-butyl ester IV:
[0047] Dissolve 93.8 g of compound III in 1.5 L of dry tetrahydrofuran and 400 mL of methanol, cool to -80°C under nitrogen protection, add 427 mL of diethylmethoxyborane (1 mol / L tetrahydrofuran solution), stir for 20 minutes, and then add Sodium borohydride 16.5g, react at this temperature for 3h, add 200mL acetone and 80mL30% hydrogen peroxide, react at -60°C for 30min, pour the reaction system into 800mL water, extract with ethyl acetate (400mL×3), and combine the organic phases , the organic phase was washed with water (100 mL×3), dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain solid IV, which was recrystallized from n-hexane to obtain 77.6 g of a pale yellow solid, with a yield of 82.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com