Preparation detection method for substances related to rosuvastatin calcium preparation
A technology of rosuvastatin calcium and rosuvastatin, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
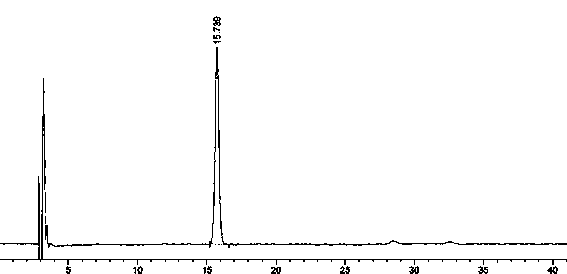
[0061] Preparation of Impurity 1, Impurity 3, and Impurity 4:
[0062] Take 0.5g of rosuvastatin calcium raw material, put it in a 50ml measuring bottle, add 2ml of 7mol / L hydrochloric acid solution, dissolve it with 50% acetonitrile solution and set the volume to the mark, shake well, place it in a water bath at 60°C for 0.5h, take it out, Let cool and use as stock solution 1.
[0063] Preparation of Impurity 2:
[0064] Take 0.5g of rosuvastatin calcium raw material, put it in a weighing bottle, heat it at 105°C for 7 days, let it cool, and use 50% acetonitrile solution to prepare rosuvastatin calcium raw material containing about 0.01g / ml per 1ml solution, as stock solution 2.
[0065] Preparation of impurity 5 and impurity 6:
[0066] Take 0.5g of rosuvastatin calcium raw material, put it in a 50ml measuring bottle, add 50% acetonitrile solution to dissolve and adjust the volume to the mark, shake well, put it under natural light for 4h, and use it as stock solution 3. ...
Embodiment 1
[0090] Instruments and reagents
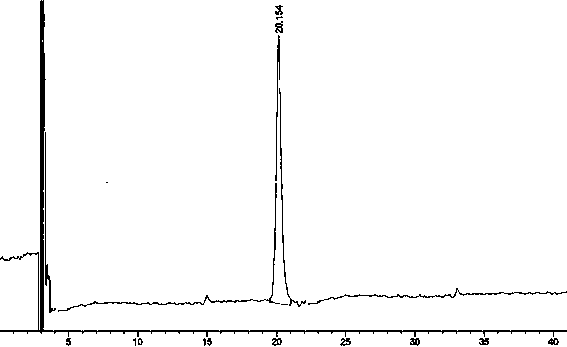
[0091] Agilent1260 series high performance liquid chromatography; Sartorius CP225D analytical balance (1 / 100,000 grade); impurity reference substances: ①(3S,5S)-Rosuvastatin calcium diastereoisomers, ②Rosuvastatin- 5S-lactone, ③ rosuvastatin-5R-lactone, ④ rosuvastatin-5-oxidation, ⑤ rosuvastatin photodegradation product 1, ⑥ rosuvastatin photodegradation product 2, ⑦ rosuvastatin Statin photodegradation product 1-lactone, ⑧ rosuvastatin photodegradation product 2-lactone, self-made, purity ≥ 95%; rosuvastatin calcium tablets (self-made): batch number: 20130826 / 10mg.
[0092] Chromatographic conditions:
[0093] Agilent Zorbax extend-C18 (4.6*250mm, 5μm); with 0.05M sodium dihydrogen phosphate buffer (adjust pH to 2.0 with phosphoric acid): acetonitrile:methanol=46:20:34; detection wavelength: 242nm; column temperature: 40 °C; flow rate: 0.7ml / min; injection volume: 10μl.
[0094] Sample Preparation:
[0095] Preparation of reference substa...
Embodiment 2
[0102] Instruments and reagents
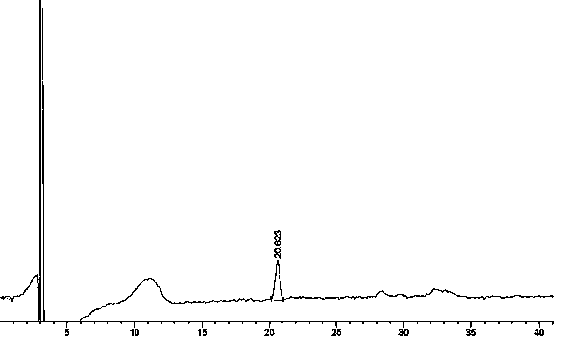
[0103] Agilent1260 series high performance liquid chromatography; Sartorius CP225D analytical balance (1 / 100,000 grade); impurity reference substances: ①(3S,5S)-Rosuvastatin calcium diastereoisomers, ②Rosuvastatin- 5S-lactone, ③ rosuvastatin-5R-lactone, ④ rosuvastatin-5-oxidation, ⑤ rosuvastatin photodegradation product 1, ⑥ rosuvastatin photodegradation product 2, ⑦ rosuvastatin Statin photodegradation product 1-lactone, ⑧ rosuvastatin photodegradation product 2-lactone, self-made, purity ≥ 95%; rosuvastatin calcium capsule (self-made): batch number: F6-144015 / 10mg.
[0104] Chromatographic conditions:
[0105] Agilent Zorbax extend-C18 (4.6*250mm, 5μm); with 0.05M sodium dihydrogen phosphate buffer (adjust pH to 2.0 with phosphoric acid): acetonitrile:methanol=46:20:34; detection wavelength: 242nm; column temperature: 40 °C; flow rate: 0.7ml / min; injection volume: 10μl.
[0106] Sample Preparation:
[0107] Preparation of reference subst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com