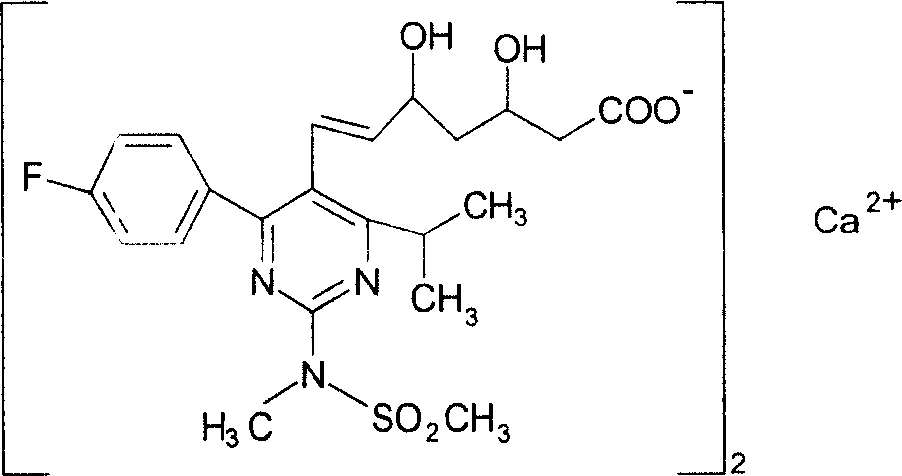
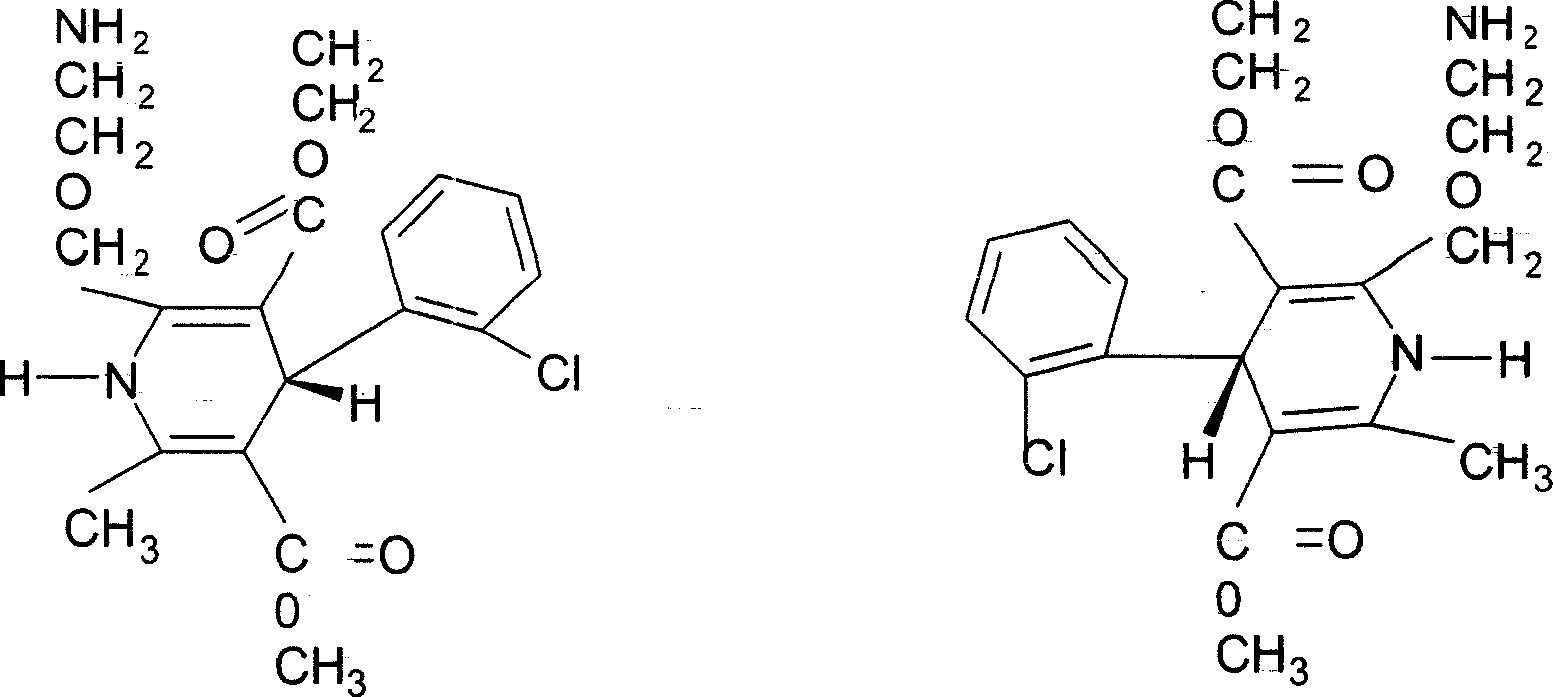
Preparations containing rosuvastatin calcium and amlodipine and method for preparing the same
A technology of rosuvastatin calcium and amlodipine is applied to the preparation field containing rosuvastatin calcium and amlodipine and the preparation field thereof to achieve the effect of synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Prescription (each tablet contains 5 mg rosuvastatin calcium: 5 mg amlodipine)
[0067] Rosuvastatin Calcium 50g
[0068] Amlodipine 50g
[0069] Starch 1500 2325g
[0070] Sodium carboxymethyl starch 2.5g
[0071] Magnesium Stearate 1.25g
[0072] Micronized silica gel 1.25g
[0073] Povidone 2.5g
[0074] Take the prescribed amount of rosuvastatin calcium and amlodipine, and mix them with the prescribed amount of starch 1500 and sodium carboxymethyl starch by using the equal amount incremental method. Gained mixture uses recipe quantity povidone solution as binding agent wet granulation (boiling granulation granulator FL3-C, Shanghai Far East Pharmaceutical Machinery Factory). The obtained wet granules were sized after drying at 55°C. Add the prescribed amount of magnesium stearate and micropowder silica gel to the granules after granulation, and then tablet (rotary tablet press ZP-19, Shanghai No. 1 Pharmaceutical Machinery Factory) to get 10,000 tablets. The...
Embodiment 2
[0076] Prescription (7.5 mg rosuvastatin calcium per tablet: 5 mg amlodipine)
[0077] Rosuvastatin Calcium 75g
[0078] Amlodipine 50g
[0079] Microcrystalline Cellulose 375g
[0080] Sucrose 750g
[0081] Calcium phosphate 1125g
[0082] Povidone 37.5g
[0083] Crospovidone 62.5g
[0085] Take the prescribed amount of rosuvastatin calcium and amlodipine, and mix them with the prescribed amount of microcrystalline cellulose, powdered sugar (powder after crushing sucrose), calcium phosphate, and crospovidone by equal addition method. Gained mixture uses recipe quantity povidone solution as binding agent wet granulation (boiling granulation granulator FL3-C, Shanghai Far East Pharmaceutical Machinery Factory). The obtained wet granules were sized after drying at 55°C. Add the prescribed amount of talcum powder to the granules after granulation, and then compress 10,000 tablets (rotary tablet press ZP-19, Shanghai No. 1 Pharmaceutical Machinery ...
Embodiment 3
[0087] Prescription (each tablet contains 9.0 mg rosuvastatin calcium: 5 mg amlodipine)
[0088] Rosuvastatin Calcium 90g
[0089] Amlodipine 50g
[0090] Microcrystalline Cellulose 375g
[0091] Starch 735g
[0092] Calcium phosphate 1125g
[0093] Povidone 37.5g
[0094] Crospovidone 62.5g
[0095] Talc powder 25g
[0096] Take the prescribed amount of rosuvastatin calcium and amlodipine, and mix them with the prescribed amount of microcrystalline cellulose, starch, calcium phosphate, and crospovidone using the equal-volume incremental method. Gained mixture uses recipe quantity povidone solution as binding agent wet granulation (boiling granulation granulator FL3-C, Shanghai Far East Pharmaceutical Machinery Factory). The obtained wet granules were sized after drying at 55°C. Add the prescribed amount of talcum powder to the granules after granulation, and then compress 10,000 tablets (rotary tablet press ZP-19, Shanghai No. 1 Pharmaceutical Machinery Factory). Gai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com