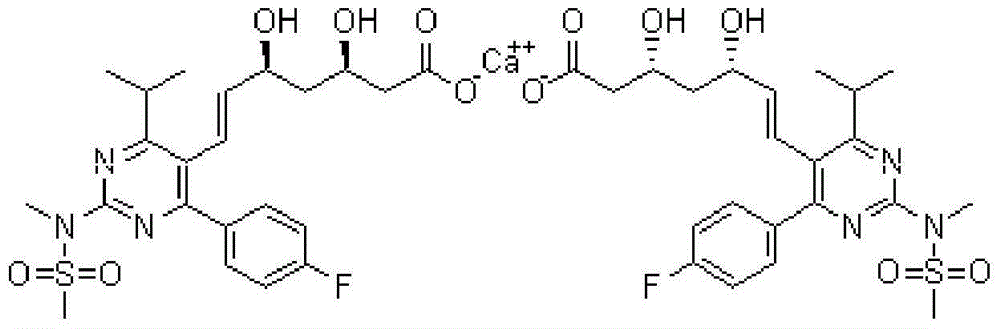
Rosuvastatin calcium tablet and preparation method thereof
A technology of rosuvastatin calcium and tablet cores, which is applied in the field of medicine, can solve the problems that the pharmaceutical composition cannot meet the storage period requirements, the pharmaceutical composition cannot be legally implemented, and the operation of the product is difficult to configure, so as to achieve excellent quality and stability Sexuality, rapid release, and the effect of solving stability problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] (1) Prescription:
[0086] Tablet prescription:
[0087]
[0088] Prescription of coating solution: dosage of 1000 tablets
[0089] Opadry 6.75g
[0090] Add purified water to 45g
[0091] (2) Preparation method:
[0092] ① Pass rosuvastatin calcium, crospovidone, and calcium hydrogen phosphate through an 80-mesh sieve, microcrystalline cellulose-lactose (1:3) compound through a 60-mesh sieve, and magnesium stearate through a 100-mesh sieve, spare.
[0093] ② Take rosuvastatin calcium, microcrystalline cellulose-lactose (1:3) complex, crospovidone, and calcium hydrogen phosphate that meet the requirements and mix them in a mixer for 45 minutes.
[0094] ③ Add magnesium stearate to the product obtained in step ② and mix evenly, measure the content of the main ingredient, determine the weight of the tablet, and compress it under the conditions of ambient temperature 18°C and humidity 45%, and the compression pressure is 15-35kN.
[0095] ④Put the tablet prepare...
Embodiment 2
[0098] (1) Prescription:
[0099] Tablet prescription:
[0100]
[0101] Prescription of coating solution: dosage of 1000 tablets
[0102] Opadry 6.75g
[0103] Add purified water to 45g
[0104] (2) Preparation method:
[0105] ① Pass rosuvastatin calcium, crospovidone, and calcium hydrogen phosphate through an 80-mesh sieve, microcrystalline cellulose-lactose (1:3) compound through a 60-mesh sieve, and magnesium stearate through a 100-mesh sieve, spare.
[0106] ② Take rosuvastatin calcium, microcrystalline cellulose-lactose (1:3) compound, and crospovidone that meet the requirements and mix them evenly in a mixer.
[0107]③ Add magnesium stearate to the product obtained in step ② and mix evenly, measure the content of the main ingredient, determine the weight of the tablet, and press it under the conditions of ambient temperature 18°C and humidity 60%, and the tableting pressure is 15-35kN.
[0108] ④Put the tablet prepared in step ③ into a coating pan for coatin...
Embodiment 3
[0111] (1) Prescription:
[0112] Tablet prescription:
[0113]
[0114] Prescription of coating solution: dosage of 1000 tablets
[0115] Opadry 6.75g
[0116] Add purified water to 45g
[0117] (2) Preparation method:
[0118] ① Pass rosuvastatin calcium, low-substituted hydroxypropyl cellulose, and tricalcium phosphate through a 80-mesh sieve, microcrystalline cellulose-lactose (1:3) compound through a 60-mesh sieve, and silicon dioxide through a 100-mesh sieve ,spare.
[0119] ② Take rosuvastatin calcium, microcrystalline cellulose-lactose (1:3) compound, and low-substituted hydroxypropyl cellulose that meet the requirements and mix them evenly in a mixer.
[0120] ③ Add silicon dioxide to the product obtained in step ② and mix evenly, measure the content of the main ingredient, determine the weight of the tablet, and press it under the conditions of ambient temperature 26°C and humidity 60°C, and the tableting pressure is 30-35kN.
[0121] ④Put the tablet prepare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com