Preparation of rosuvastatin
A technology of rosuvastatin and compounds, applied in the field of preparation of rosuvastatin intermediates, capable of solving problems such as reduction of cholesterol formation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
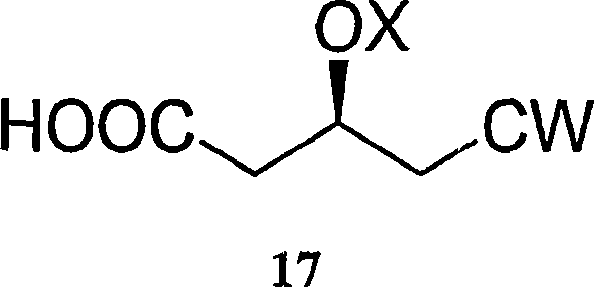
[0141] Embodiment 1: the preparation of compound 17TB
[0142]
[0143] In a 1-liter flask equipped with a condenser, mechanical stirrer, pH meter and thermometer, TBDMA-protected tert-butylethylglutaric acid (100 g, 288 micromol) and pure ethanol (500 mL) were added to form reaction mixture. The reaction mixture was heated to 50°C, 1N NaOH (115.2 mL) was added dropwise, and the measured pH was 12.8.
[0144] After 1 hour at this temperature, the pH was measured to be 10.59. Additional 1N NaOH (115.2 mL) was added. The pH was measured to be 12.25. After 1 hour, additional 1N NaOH (115.2 mL) was added.
[0145] The reaction mixture was kept at 50 °C for 7 hours until no starting material was detectable by TLC. The reaction mixture was cooled to room temperature and evaporated to a final volume of 300 mL. H 2 O (400 mL) and ethanol (95%, 50 mL) were added to the reaction mixture. The reaction mixture was washed with hexane (300 mL each).
[0146] Toluene (300 mL) was ...
Embodiment 2
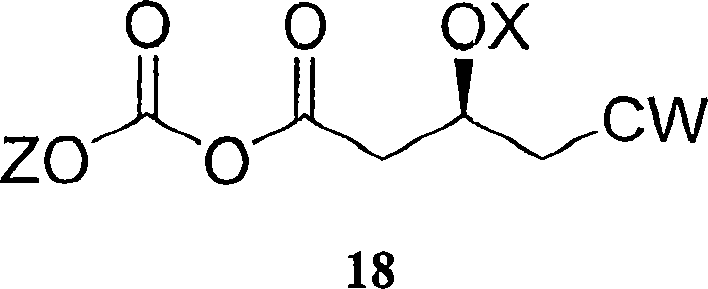
[0147] Embodiment 2: the preparation of compound 18TB
[0148]
[0149] A first solution of ethyl chloroformate (16.44 ml) in 900 ml of dry toluene (KF = less than 0.01%) was added to a 2 liter flask and the solution was cooled to -45°C. Compound 17TB (50 g) and Et 3 A second solution of N (26.06 mL) in 100 mL of toluene was added dropwise to the first solution such that the temperature of the reaction mixture was maintained at -45°C to -40°C.
[0150] The reaction mixture was slowly heated to 0°C over 1.5 hours, then quenched with water. The reaction mixture was immediately transferred to a 2-L separatory funnel and washed with NaHCO 3 (saturated, 250 ml) and NaCl (saturated, 250 ml) washed the organic layer with MgSO 4 dry. The solvent was evaporated and the residue was used in the next step without any purification step.
Embodiment 3
[0151] Embodiment 3: the preparation of compound 19TBPH
[0152]
[0153] Methyltriphenylphosphonium bromide (224.3 g) was suspended in THF (600 ml), and butyl lithium (1.6 M, 392.5 ml) was added at a temperature of about -55 to -50° C. over 30 minutes. The reaction mixture was then heated to about 0°C over a period of 1.5 hours and then cooled to about -60°C.
[0154] A solution of anhydrous compound 18TB (122.6 g, 314 mmol) in toluene (360 mL) was added dropwise to the reaction mixture for about 2 hours while maintaining the temperature of the reaction mixture at about -55 to -65°C. The reaction mixture was heated to about 0°C for 1.5 hours and quenched with water (250 mL). The aqueous phase was separated and the product was extracted from the aqueous phase with toluene (100 mL). The two organic layers were mixed together and washed with NaHCO 3 (sat, 2 x 100 mL) and NaCl (2 x 100 mL) washes. Store the organic phase in NaSO at about -25 °C 4 overnight, and the solven...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com