Method for preparing rosuvastatin intermediate
A liquid, ampicillin technology, applied in the field of biological preparation of 3-hydroxyglutaric acid monomethyl ester, can solve the problem of not meeting green chemistry and atom economy, not having industrial application value, low substrate dosage, etc. problems, to achieve the effect of mild conditions, easy operation and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 recombinant nitrilase
[0028] Inoculate a single colony of recombinant Escherichia coli containing the nitrilase gene from a glycerol tube or transformation plate into 4 mL of liquid LB medium containing ampicillin resistance and activate overnight (37°C, 200rpm). Transfer 100mL liquid LB medium containing ampicillin resistance from the overnight culture at 1 / 100 inoculum size, culture at 37°C, 200rpm shaking until OD 600 When the value reaches 0.6-0.8, add IPTG and continue culturing overnight at 30°C. Cells were collected by centrifugation, and suspended in 10 mL of phosphate buffer (2 mM, pH 7.0). The cell suspension was ultrasonically broken in an ice bath for 10 minutes, centrifuged, the supernatant was pre-frozen overnight, and freeze-dried for 24h-48h to obtain the recombinant nitrilase in the form of freeze-dried powder.
Embodiment 2
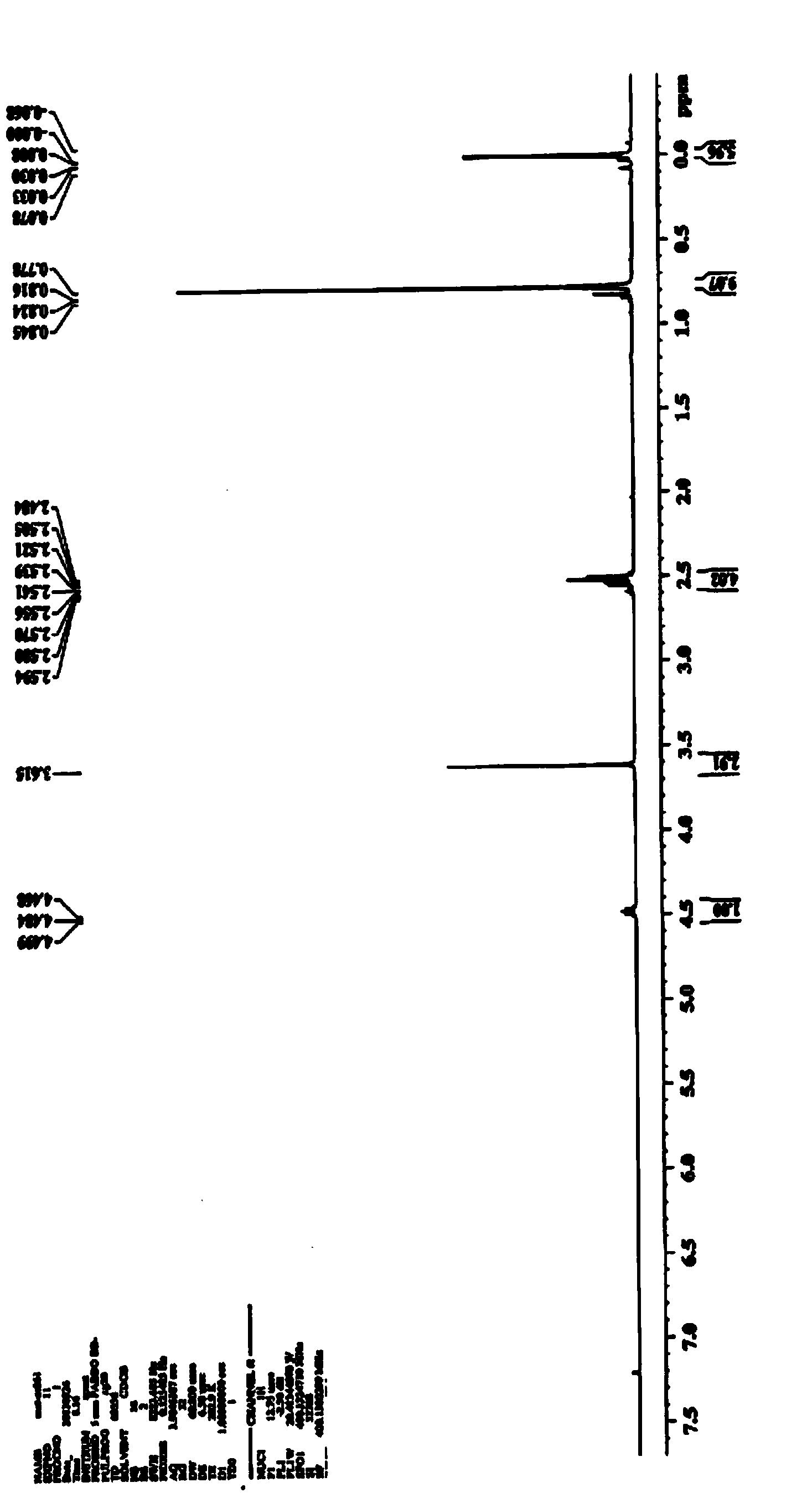
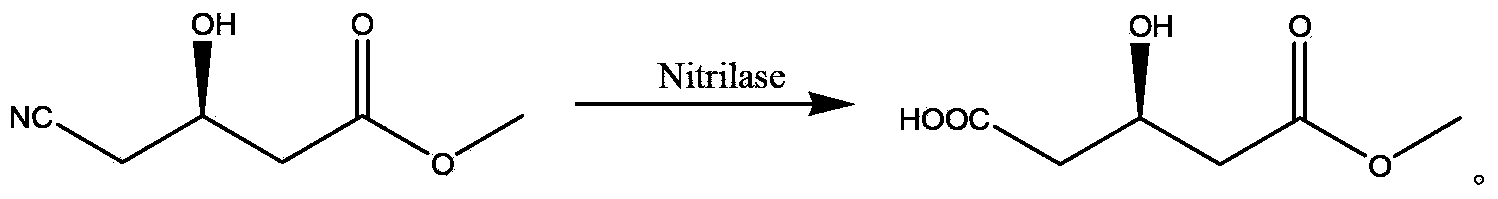
[0029] The preparation of embodiment 2 gram level (R)-3-hydroxyglutaric acid monomethyl ester
[0030] Add 85ml of phosphate buffer (0.1M, pH8.0) to a 250mL round bottom flask, add 450mg of recombinant nitrilase; weigh 15g of (R)-methyl 4-cyano-3-hydroxybutyrate and use Add a constant current pump to the system, stir the reaction at 30°C for 24 hours, then take a sample for LC-MS analysis, the conversion rate is >99%, stop the reaction, adjust the pH of the reaction solution to acidic (pH=2~3) with hydrochloric acid, Filter through diatomaceous earth, rinse the filter residue with ethyl acetate; extract the filtrate with ethyl acetate, combine the organic phases; dry over anhydrous sodium sulfate, and concentrate to obtain 14g of a yellow-brown oily substance (R)-3-hydroxyglutaric acid mono Methyl ester, yield 81.4%, GC purity 88.2%.
Embodiment 3
[0031] Preparation of 3 kilograms of embodiment (R)-3-hydroxyglutaric acid monomethyl ester
[0032] Add 8.5L phosphate buffer (0.1M, pH8.0) in the reactor, then add 45g recombinant nitrilase; Weigh 1.5Kg (R)-4-cyano-3-hydroxybutyrate methyl ester and adopt A constant flow pump was added to the system, and the reaction was mechanically stirred at 30°C for 24 hours, and then a sample was taken for LC-MS analysis. The conversion rate was >99%, and the reaction was terminated. Adjust the pH of the reaction solution to acidity (pH=2~3) with hydrochloric acid, filter with diatomaceous earth, wash the filter residue with ethyl acetate; extract the filtrate with ethyl acetate, combine the organic phases; dry with anhydrous sodium sulfate, and concentrate to obtain about 1.4Kg The yellow-brown oil is (R)-3-hydroxyglutaric acid monomethyl ester, the yield is 75%, and the GC purity is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com