Method for preparing Rosuvastain and its intermediate
A technology of rosuvastatin and asana, which is applied in a new field of preparation, can solve the problems of difficulty, high cost, and low yield in the synthesis of phosphonate esters, and achieve the goals of high product quality, increased yield, and increased overall yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
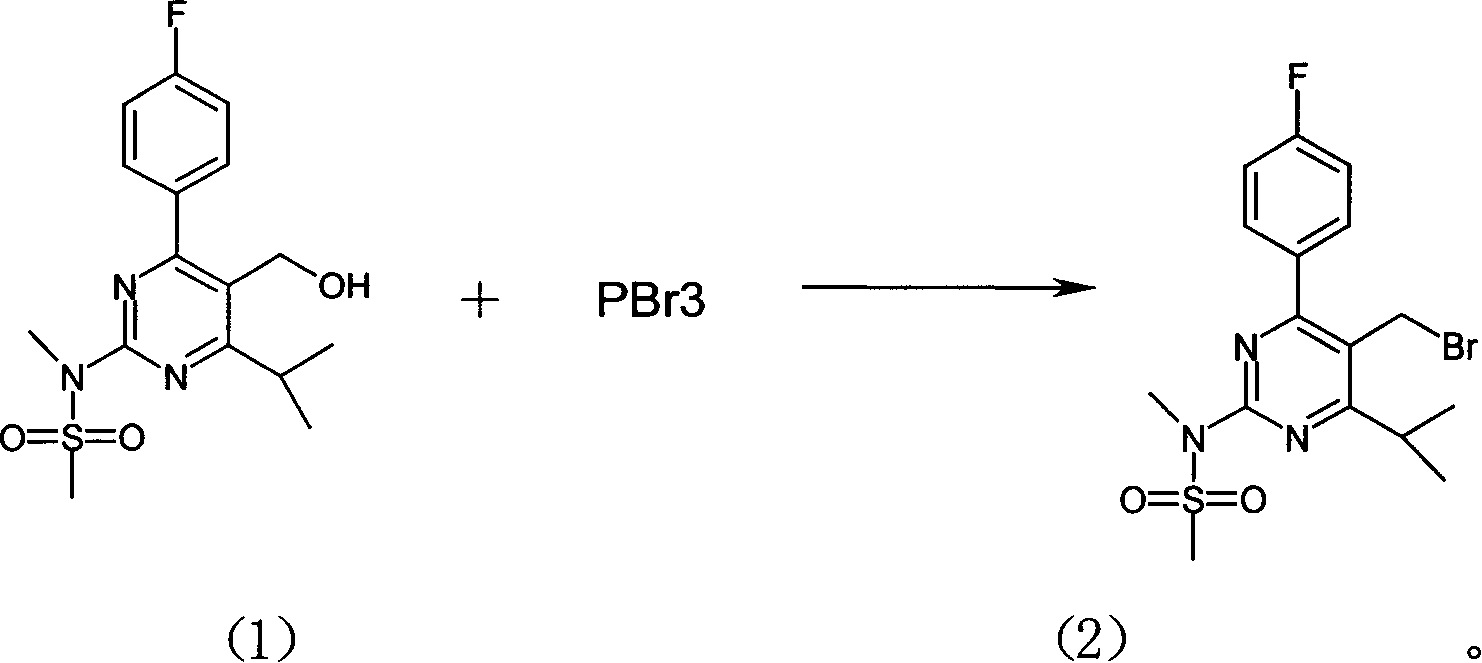
[0022] Example 1: Synthesis of 4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl-amino)-pyrimidin-5-yl-bromomethane ( Preparation of compound (2))
[0023] In a 2-liter four-necked flask, 143.3 g of compound (1) (see EP0521471A for its preparation method) was put in, 780 ml of dichloromethane was added and stirred to dissolve, and 780 ml of toluene was added. Stir for another 10 minutes. 63ml of phosphorus tribromide was added dropwise, and the temperature was controlled at <0°C. After the dropwise addition was completed, the reaction was kept at 0°C for 3 hours. The reaction solution was poured into the prepared 1200ml saturated sodium bicarbonate solution, stirred for 10 minutes, allowed to stand to separate layers, and the organic layer was extracted. Wash once with 350ml of water, add 180g of anhydrous magnesium sulfate to dry for more than 5 hours, filter, concentrate under reduced pressure, add 300ml of n-hexane, stir and crystallize at room temperature, filt...
Embodiment 2
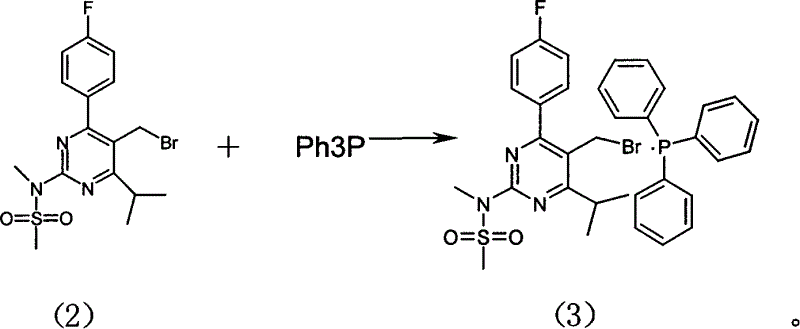
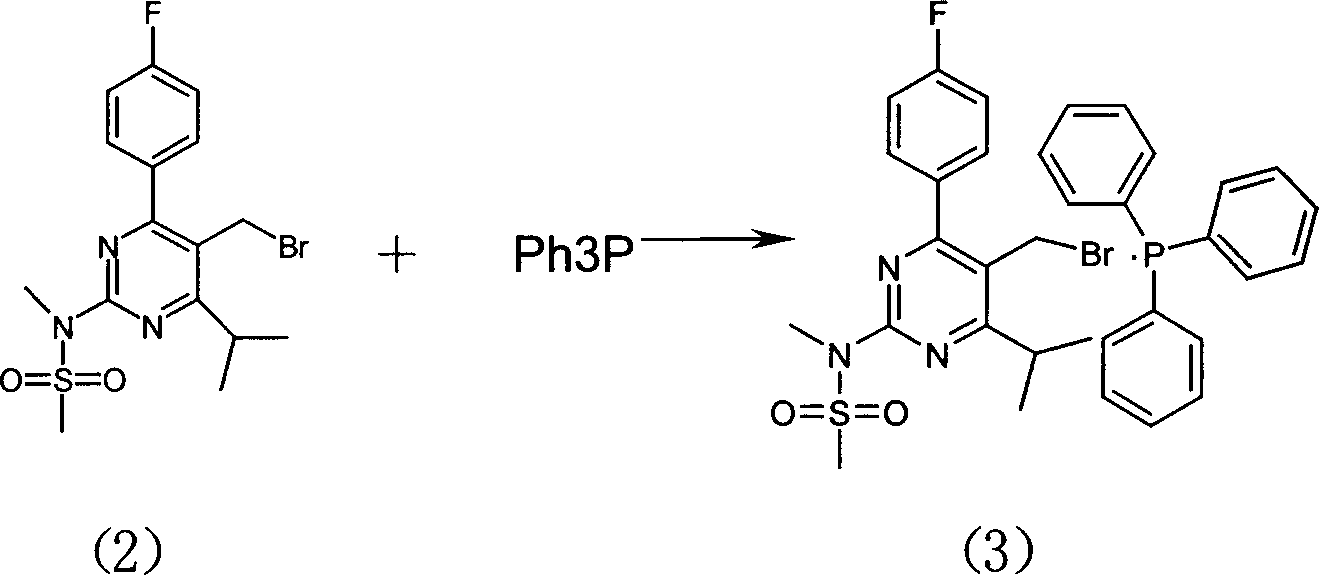
[0024] Example 2: 4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl-amino)-pyrimidin-5-yl-bromomethanetriphenyl Preparation of Phosphonium Salt (Preparation of Compound (3))
[0025] Reaction at room temperature, 261.8g of compound (2) was dissolved in 1730ml of toluene. Slowly add 420 ml of toluene solution containing 130 g of triphenylphosphine dropwise at room temperature, complete the addition in about 5 hours, and stir overnight. After centrifugal filtration, the solid was slurried once with 760ml of toluene, centrifugally filtered, and dried to obtain 284.4g of white solid (compound (3)), with a yield of 81%.
Embodiment 3
[0026] Example 3: (E)-4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl-amino)-pyrimidin-5-yl-( Preparation of 3R, 5S)-2,2-dimethyl-1,3-dioxolane-4-acetic acid tert-butyl ester (preparation of compound (5))
[0027] In a 500ml four-neck flask, add 50g of compound (3), 18.5g of side chain aldehyde (4) (prepared according to the method of Example 6 of US4970313A), 400ml of tetrahydrofuran, heat to 70°C, and stir to dissolve. Heating was stopped, and 2.75 g of 60% sodium hydride was added. After stirring for 15 minutes, the reaction solution was poured into ice water to terminate the reaction. Add 950ml of ethyl acetate to extract the organic layer, wash twice with 200ml of water, add 70g of anhydrous sodium sulfate and dry for more than 5 hours. Filter and concentrate to dryness under reduced pressure. Add 480ml of ethanol and cool to -5°C for crystallization for 6 hours. Filter and drain to obtain the crude product, which is dissolved and recrystallized by heating...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com