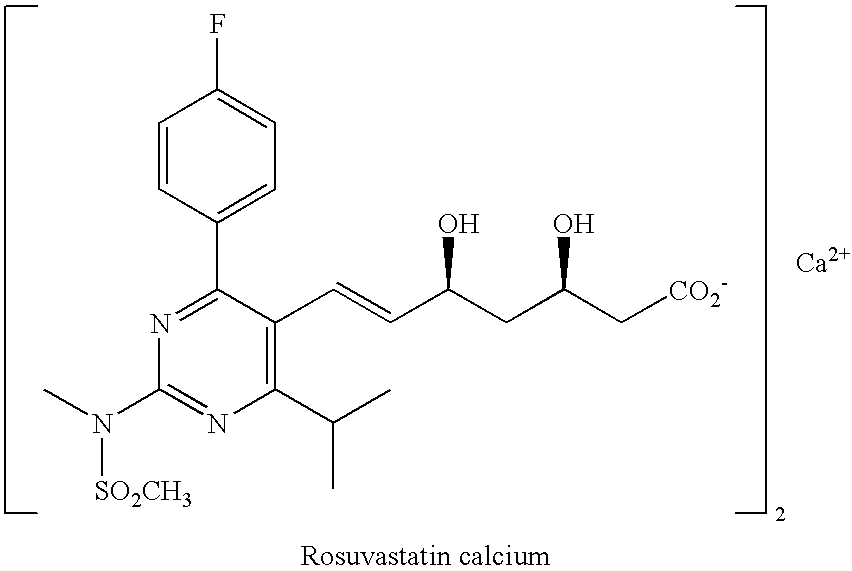
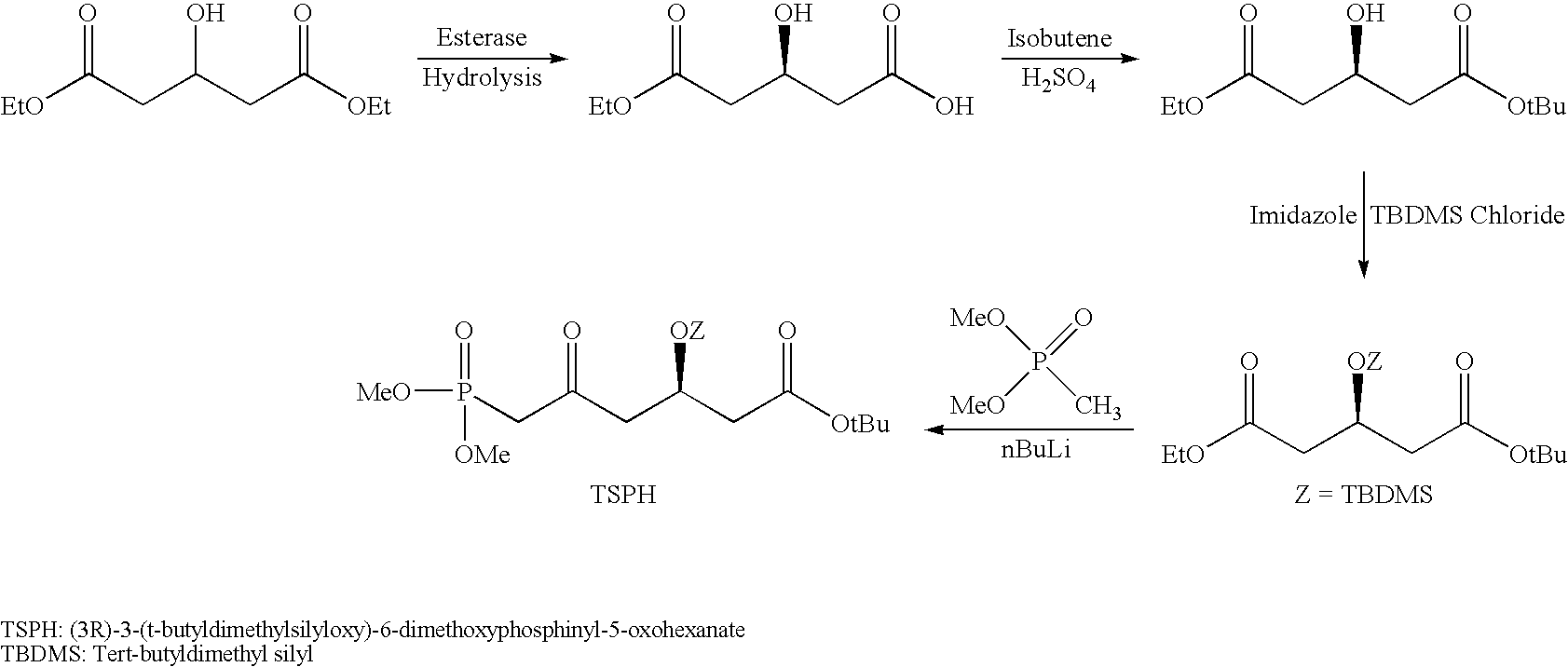
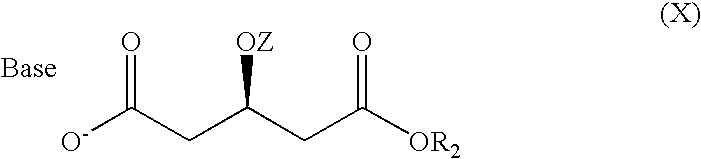
Rosuvastatin intermediates and process for the preparation of rosuvastatin
a technology of rosuvastatin and rosuvastatin, which is applied in the field of rosuvastatin intermediates and process for the preparation of rosuvastatin, can solve the problems of difficult purification of tsph by column chromatography, process problems, and unsatisfactory industrial processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 3-Hydroxy Dihydrogen Glutarate (TBDMS Protected) (Protected Diacid) from 3-Hydroxy Diethyl Glutarate (TBDMS Protected) Through Hydrolysis (Formula V from Hydrolysis of Formula IV)
[0117]To a 5-liter four neck round bottom flask under nitrogen atmosphere added 163 g 3-hydroxy diethyl glutarate (TBDMS protected) and 815 ml methanol at 25 to 30° C., and added 45.10 g sodium hydroxide with 326 ml water to the reaction mass over the period of 1 hr at 30 to 32° C. Stirred reaction mass at 30 to 32° C. Starting material was not detected after 24 hr by TLC. White colored disodium salt was observed. Added 500 g 10% hydrochloric acid solution to adjust pH of reaction mass to 3.5 to produce a clear solution. Added 275 g sodium chloride to saturate reaction mass and extracted product in 4×650 ml dichloromethane, evaporated solvent under vacuum at 40 to 50° C. to get white colored crude product which was further stirred with 600 ml hexane and filtered. Dry the product under vacuum ...
example 2
Preparation of 3-Hydroxy Glutaric Anhydride (TBDMS Protected) from 3-Hydroxy Dihydrogen Glutarate (TBDMS Protected) (Formula VI from Formula V)
[0119]To a 2-liter four neck round bottom flask under nitrogen atmosphere added 180 g solid 3-hydroxy dihydrogen glutarate (TBDMS protected) and 630 ml acetic anhydride at 25 to 30° C. and reflux reaction mass for 2 hr at 130-135° C. Monitored by TLC and GC, starting material was not found in 2.0 hr. Acetic anhydride was distilled under vacuum at 75-80° C. completely and crude product was crystallized through 180 ml hexane and dried pure brown colored product under vacuum at 40° C. Obtained 171 g pure product.
[0120]Yield 100% purity 99.5% (Area % by GC)
example 3
Preparation of Racemic Methyl Hydrogen 3-Hydroxy Glutarate (TBDMS Protected) From 3-Hydroxy Glutaric Anhydride (TBDMS Protected) (Formula VII from Formula VI)
[0121]To a 2-liter four neck round bottom flask under nitrogen atmosphere added 20 g 3-hydroxy glutaric anhydride (TBDMS protected), 200 ml methanol and reflux reaction mass for 12 hr at 60 to 65° C. Monitored reaction progress by GC, starting material was not detected. Allowed reaction mass temperature up to room temperature (“RT”) and added 2-3 drop of hydrochloric acid and 100 ml water. Added sodium chloride to saturate reaction mass at room temperature. Extracted product with 200 ml dichloromethane. Aqueous layer was extracted with 20 ml dichloromethane and combined organic layer was dried over sodium sulphate. Solvent was evaporated to give 20.0 g product.
[0122]Yield 88.4% purity 94.5% (Area % by GC)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com