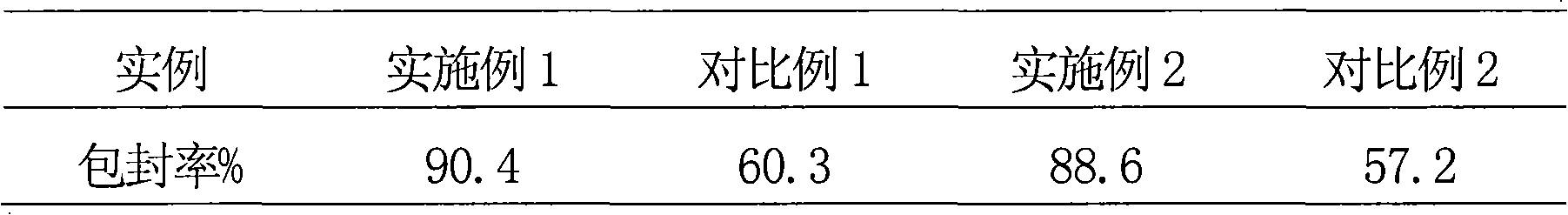
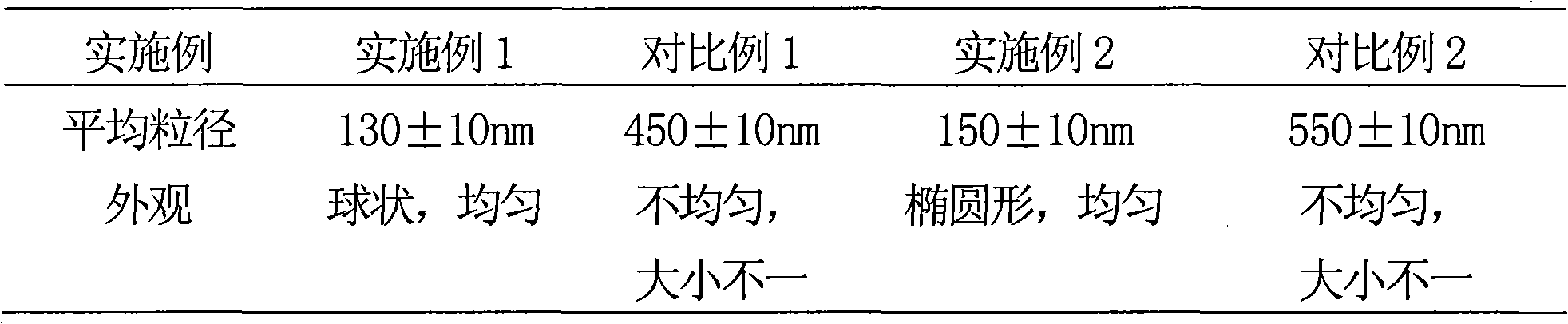
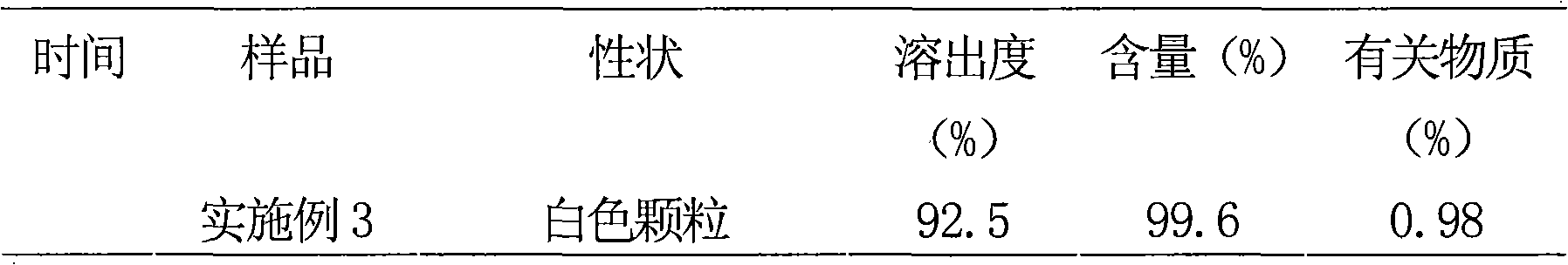
Solid preparation containing rosuvastain calcium liposome
A technology of rosuvastatin calcium and liposome preparations, which is applied in the field of medicine, can solve the problems that the pharmaceutical composition does not meet the storage period requirements, the difficulty in preparing products, and the reduction of bioavailability, so as to protect vascular endothelial cells and microvascular system, reducing the size of myocardial infarction, and enhancing the effect of targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Implementation Example 1 Preparation of Rosuvastatin Calcium Liposome
[0055] Prescription Rosuvastatin Calcium 100g
[0056] Soy Lecithin 800g
[0057] Cholesterol 300g
[0058] Tween 80 50g
[0059] Sodium deoxycholate 100g
[0060] Preparation Process
[0061] (1) Dissolve 100g rosuvastatin calcium, 800g soybean lecithin, 300g cholesterol, 50g Tween 80 and 100g sodium deoxycholate in 5000ml methanol, mix well, remove methanol under reduced pressure on a rotary thin film evaporator, Prepare phospholipid film;
[0062] (2) Add 2500ml of phosphoric acid-sodium dihydrogen phosphate buffer solution with pH = 4.5, stir to completely hydrate the phospholipid membrane, then homogeneously emulsify with a tissue grinder for 20min at a speed of 12000r / min, mix the solution evenly, and keep warm at 50°C Under ultrasonic treatment for 60 minutes, the liposome suspension was obtained;
[0063] (3) Spray-dry the suspension obtained in the ...
Embodiment 2
[0070] Example 2 Preparation of Rosuvastatin Calcium Liposome
[0071] Prescription: Rosuvastatin Calcium 200g
[0072] Soy Lecithin 5000g
[0073] Cholesterol 2000g
[0074] Tween 80 2400g
[0075] Sodium deoxycholate 2000g
[0076] Preparation Process
[0077] (1) 200g rosuvastatin calcium, 5000g soybean lecithin, 2000g cholesterol, 2400g Tween 80 and 2000g sodium deoxycholate were dissolved in 40000ml methanol, mixed uniformly, and decompressed on a rotary thin film evaporator to remove methanol, Prepare phospholipid film;
[0078] (2) Add 20000ml of citric acid-sodium citrate buffer solution with pH = 6.0, stir to completely hydrate the phospholipid membrane, then homogeneously emulsify for 10min with a tissue grinder at a speed of 15000r / min, mix the solution evenly, and keep warm for 70 Ultrasound at ℃ for 40 minutes to prepare liposome suspension;
[0079] (3) Spray-dry the suspension obtained in the above step (2) to prepare rosuvasta...
Embodiment 3
[0091] Example 3 Preparation of Rosuvastatin Calcium Liposome Particles
[0092] Prescription (1000 bags)
[0093] Rosuvastatin calcium liposome (calculated as rosuvastatin calcium) 10g
[0094] Starch 80g
[0095] Dextrin 35g
[0096] Sucrose 50g
[0097] Aspartame 1.5g
[0098] Hypromellose 1.2g
[0099] Strawberry essence 1.5g
[0100] Preparation Process
[0101] (1) Pulverize the liposome containing 10g rosuvastatin calcium, cross 80 mesh sieves, and set aside;
[0102] (2) Take by weighing 80g starch, 35g dextrin, 50g sucrose and 1.5g aspartame, cross 80 mesh sieves, mix and set aside;
[0103] (3) Mix the above raw and auxiliary materials evenly, then mix evenly with 1.5g strawberry essence, add 60ml of 2% hypromellose 20% ethanol solution to make soft material, pass through a 20 mesh sieve to granulate, dry at 60°C, 18 mesh Sieve whole grains, set aside;
[0104] (4) Divide the dried granules to prepare rosuvastatin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com