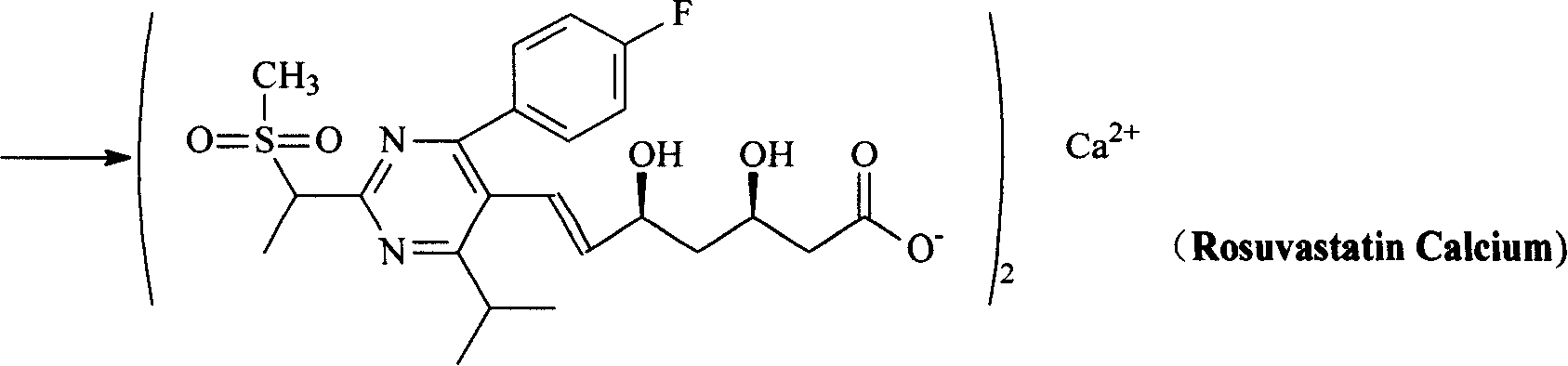
Preparation method and intermediate of rosuvastatin and its pharmaceutical salts
The technology of a medicinal salt and a new method is applied in the field of preparation and intermediates of rosuvastatin and medicinal salts thereof, and can solve the problems of long steps, high cost, low total yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
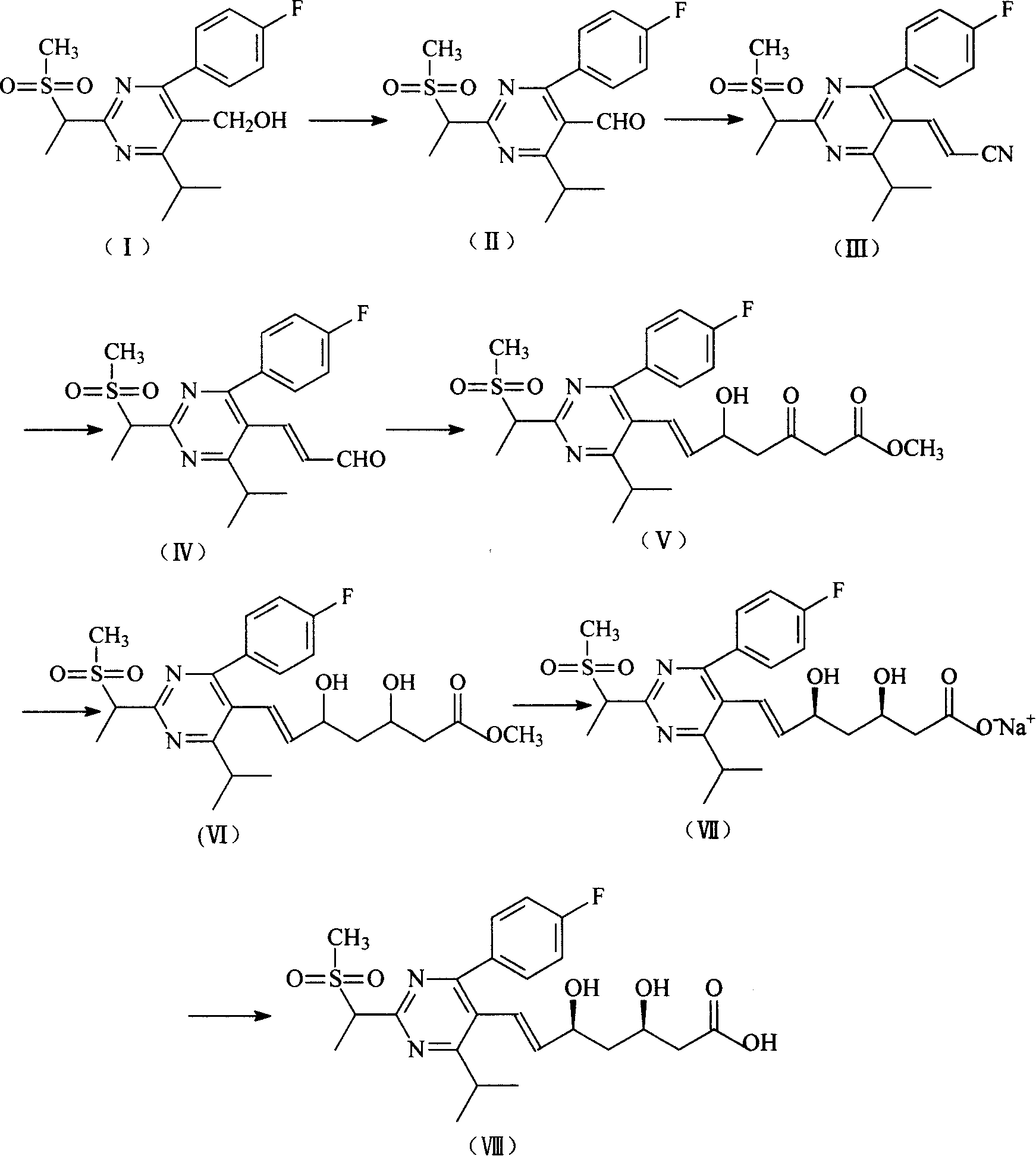
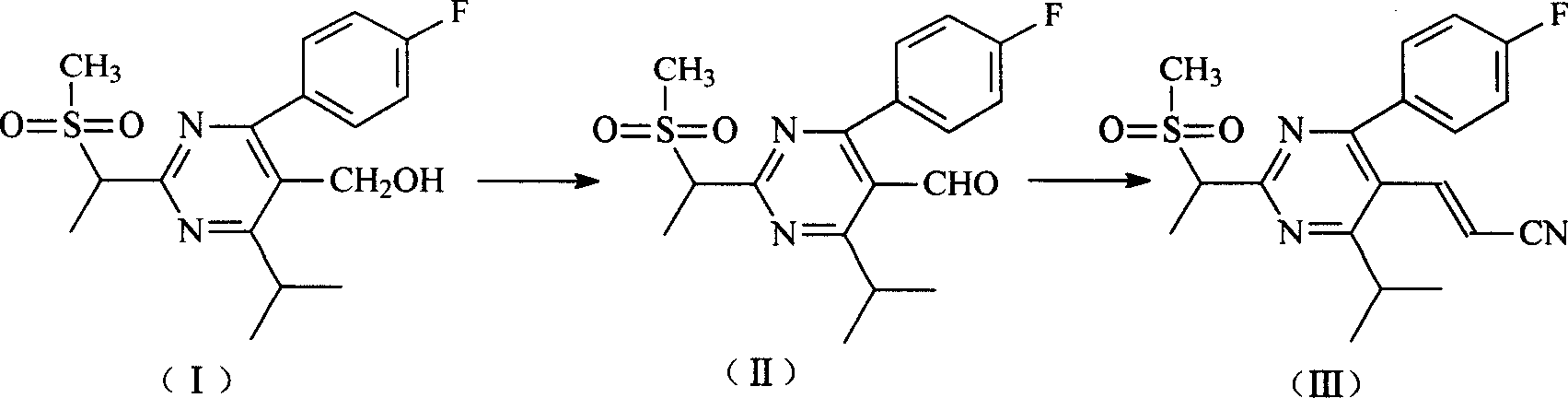
[0027] Example 1 Preparation of [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)-5-pyrimidine]carbaldehyde (II).
[0028] Add 6g [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino) to the suspension of 5.4gPCC in dichloromethane (25ml) -5-pyrimidine]methanol (I) in dichloromethane (37ml), stirred at room temperature for 2h, poured into a mixture of EA (200ml) and water (200ml), shaken and separated into layers, and the organic phase was washed twice with water. Wash with saturated sodium chloride solution once and concentrate under reduced pressure to obtain 5.5 g of gray crystals [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)- 5-pyrimidine) formaldehyde (II). 1 H-NMR(CDCl 3 , 400MHz): 9.97(S.1H), 7.64(m.2H), 7.26(m.2H), 4.02(m.1H), 3.64(s.3H), 3.56(s.3H), 1.33(d. 3H), 1.31 (d.3H).
Embodiment 2
[0029] Example 2 [4-(4-Fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)-5-(1-propenylnitrile)]pyrimidine (III) Preparation
[0030] Add 25ml of toluene to 6g [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)-5-pyrimidine]formaldehyde (II) (17mol) After stirring at room temperature to dissolve, 5.8ml (18.6mol) of cyanomethyl diethyl phosphate and 0.2ml of Aliquat 336 (0.40mmol, 3% in (II)) were added. While stirring, 10ml of 5N sodium hydroxide solution was added dropwise for 2h. Stir for another 2h after dripping. Add water to terminate the reaction, separate the layers, wash the organic layer with water until it is neutral, and concentrate under reduced pressure to obtain 5.6 g of light yellow crystals [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N) -Methanesulfonylamino)-5-(1-propenylnitrile)]pyrimidine (III) (molar yield: 88%). 1 H-NMR(CDCl 3 , 400MHz): 7.58(m.2H), 7.52(d.1H), 7.19(m.2H), 5.30(d.1H), 3.60(s.3H), 3.53(s.3H), 3.30(m. 1H), 1.33(d.3H), ...
Embodiment 3
[0031] Example 3 [4-(4-Fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)-5-(1-acrylaldehyde)]pyrimidine (IV) preparation
[0032] 4.5g [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)-5-(1-propenylnitrile)]pyrimidine(III)( 12mol), dissolved in 50ml of toluene, cooled to about -50°C, dripped 24ml (24mmol, 2 times) of diisobutylaluminum hydrogen toluene solution (1M), dripped for more than 2h, and kept for 2h after dripping. Then add 0.8ml methanol and stir for 40min, then add 3.5ml 1N hydrochloric acid solution and stir for 1.5h. Separate the layers, wash with water, NaHCO3, and concentrate to obtain 4.0g of white crystals [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)-5 -(1-Acrolein)]pyrimidine (IV) (molar yield 88.2%).1 H-NMR(CDCl 3 , 400MHz): 9.62(d.1H), 7.71(m.2H), 7.58(m.2H), 7.59(dd.1H), 6.20(dd.1H), 3.60(s.3H), 3.52(s. 3H), 3.39(m.1H), 1.33(d.3H), 1.31(d.3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com