Application of system for detecting miR-30c-5p expression quantity in prediction of curative effect of aspirin for treating cardiovascular disease patients
An aspirin and expression technology, applied to a system for detecting the expression of miR-30c-5p in the application field of predicting the efficacy of aspirin on patients with cardiovascular disease, can solve problems such as poor consistency and insufficient predictive value of aspirin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, be used for predicting the determination of aspirin to patients with cardiovascular and cerebrovascular diseases
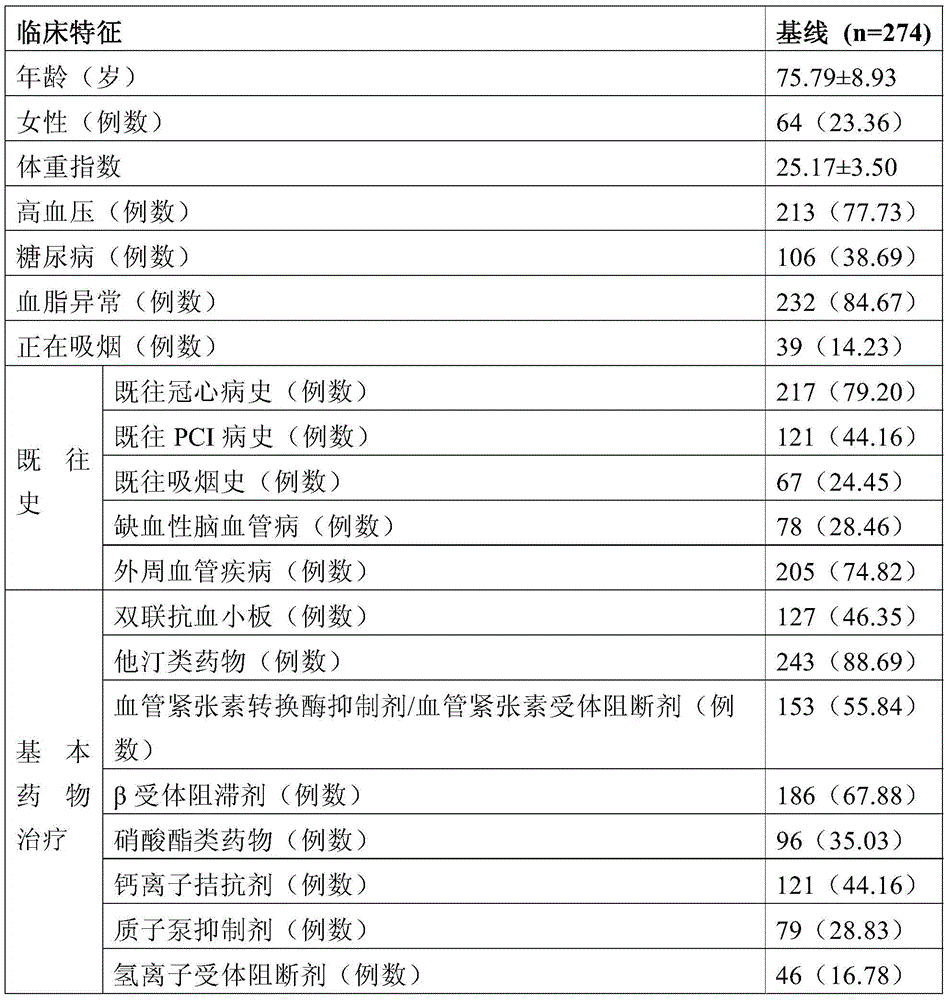
[0030] 350 patients with high cardiovascular risk, the inclusion criteria are as follows: age ≥ 50 years; patients with at least one of the following cardiovascular disease risk factors: stable angina, acute coronary syndrome (ACS), percutaneous coronary intervention (PCI) surgery, coronary artery bypass graft (CABG) surgery and ischemic cerebrovascular. These 350 patients with high cardiovascular risk were obtained after excluding some patients according to the exclusion criteria. The exclusion criteria were one of the following conditions: platelet count less than 100×10 9 / L; blood system diseases; application of GPIIb / IIIa antagonists, warfarin or non-steroidal anti-inflammatory drugs; severe liver and kidney insufficiency; contraindications to aspirin use.
[0031] The clinical data of the selected patients were collected: demographic da...
Embodiment 2
[0043] Example 2. Screening of gene markers for predicting the clinical curative effect of aspirin on patients with cardiovascular and cerebrovascular diseases
[0044] Among the 40 patients who regularly took aspirin after coronary stenting, 20 typical patients who still had cardiovascular events after regular use of 100 mg / d aspirin antiplatelet therapy after coronary stenting were selected, named as the cardiovascular event group; The matched 20 patients who regularly used 100 mg / d aspirin antiplatelet therapy after coronary stenting without cardiovascular events were selected as the control group. There were no significant statistical differences in age, sex, body mass index, smoking, hypertension, hyperlipidemia, and previous myocardial infarction between the cardiovascular event group and the control group (P>0.05); There was no statistical difference in drug treatment and other aspects (P>0.05).
[0045] Quantitative PCR analyzer 7500 system was used to quantitatively ...
Embodiment 3
[0081] Example 3. Verification of microRNA markers used to predict the clinical efficacy of aspirin on patients with cardiovascular and cerebrovascular diseases
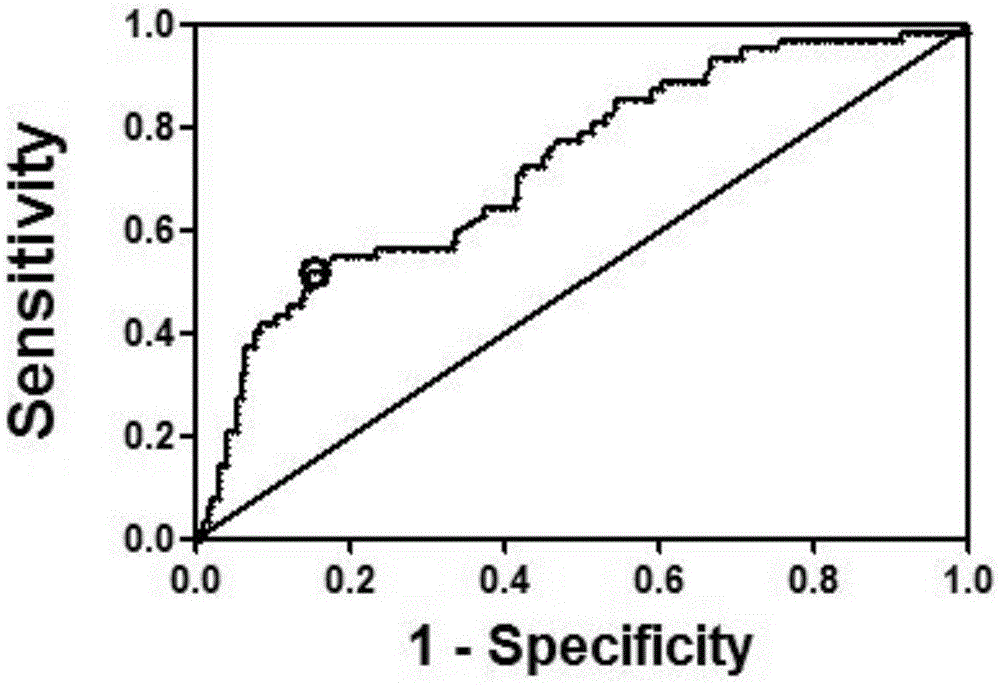
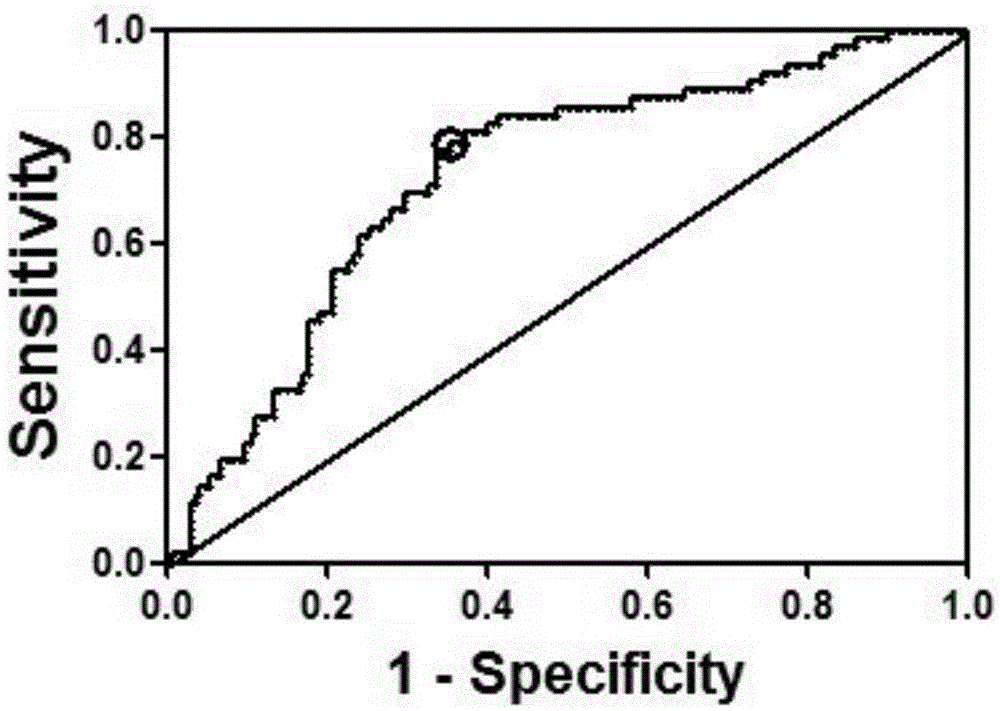
[0082] According to the detection method of platelet aggregation rate in Example 1, 310 cases of selected patients for the verification study of fluorescent quantitative PCR after microRNA screening were divided into groups, including 74 patients in the HAPR group and 236 patients in the No-HAPR group.
[0083] Whole blood microRNA was extracted from 310 patients selected for the validation study of fluorescent quantitative PCR after microRNA screening, and the extracted whole blood microRNA was reverse-transcribed and stored at -20°C; 10 microRNAs were amplified, and the relative expression levels of these 10 microRNAs relative to U6 in the HAPR group and the No-HAPR group were quantitatively analyzed (Table 5). Correlation of curative effect.
[0084] Table 5. Relative expression of 10 microRNAs in HRP and No-HPR gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com