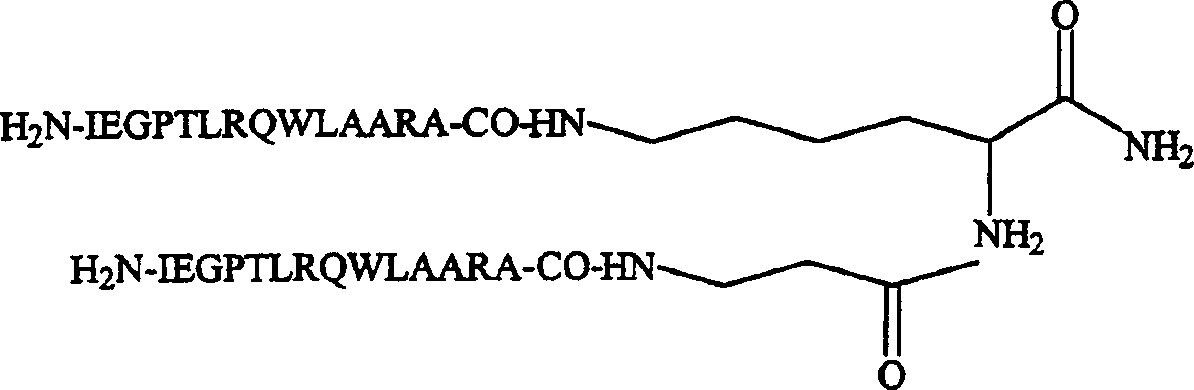
Dimeric thrombopoietin peptide mimetics binding to MP1 receptor and having thrombopoietic activity
A technology of receptor binding and compounds, applied in the field of compounds, can solve problems such as lack of sequence homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0098] Lysine and amino terminal residues can be reacted with succinic acid or other carboxylic anhydrides. The effect of derivatization reactions with these reagents is to reverse the charge of the lysine residues. Other suitable reagents for derivatizing residues containing α-amino groups include: imidate such as methyl picolinimidate; pyridoxal phosphate; pyridoxal; chloroboration trinitrobenzenesulfonic acid; O-methylisourea; 2,4-pentanedione; and transaminase-catalyzed reactions with glyoxylate.
[0099]Arginine residues can be modified by reaction with one or several conventional reagents, including phenylacetaldehyde, 2,3-butanedione, 1,2-cyclohexanedione, and ninhydrin. The derivatization of arginine residues requires basic conditions due to the high pKa of the guanidine functional group. Furthermore, these reagents react with both lysine and arginine guanidine groups.
[0100] The specific modification of tyrosine residues has been intensively studied, with particu...
Embodiment
[0237] Ⅰ. Examples of preparation methods for some of the first group of compounds disclosed herein will be described below.
[0238] A. Materials and Methods
[0239] All amino acid derivatives (all in L configuration) and resins used in peptide synthesis were purchased from Novabiochem. Peptide synthesis reagents (DCC, HOBt, etc.) were purchased from Applied Biosystems, Inc. in solution. Both PEG derivatives were purchased from Shearwater Polymers, Inc. All solvents (dichloromethane, N-methylpyrrolidone, methanol, acetonitrile) were purchased from EM Sciences. HPLC analysis was performed on a Vydac column (0.46cm×25cm, C18 reverse phase, 5mm) of the Beckman system with an elution flow rate of 1 ml / min and dual UV detection at 220 and 280 nm. A linear gradient of two mobile phases, buffer A-water (0.1% TFA) and buffer B-acetonitrile (0.1% TFA) was used in all HPLC runs. The numbered peptides cited here, such as 17b, 18, 19 and 20, may be referred to in Table 1 for numer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com