Preparation and application of dimerized fusion protein
A fusion protein and doublet technology, applied in the direction of albumin peptide, fusion polypeptide, serum albumin, etc., can solve the problems of neutralizing antibody, failure to detect thrombopoietic activity, and inhomogeneous target
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
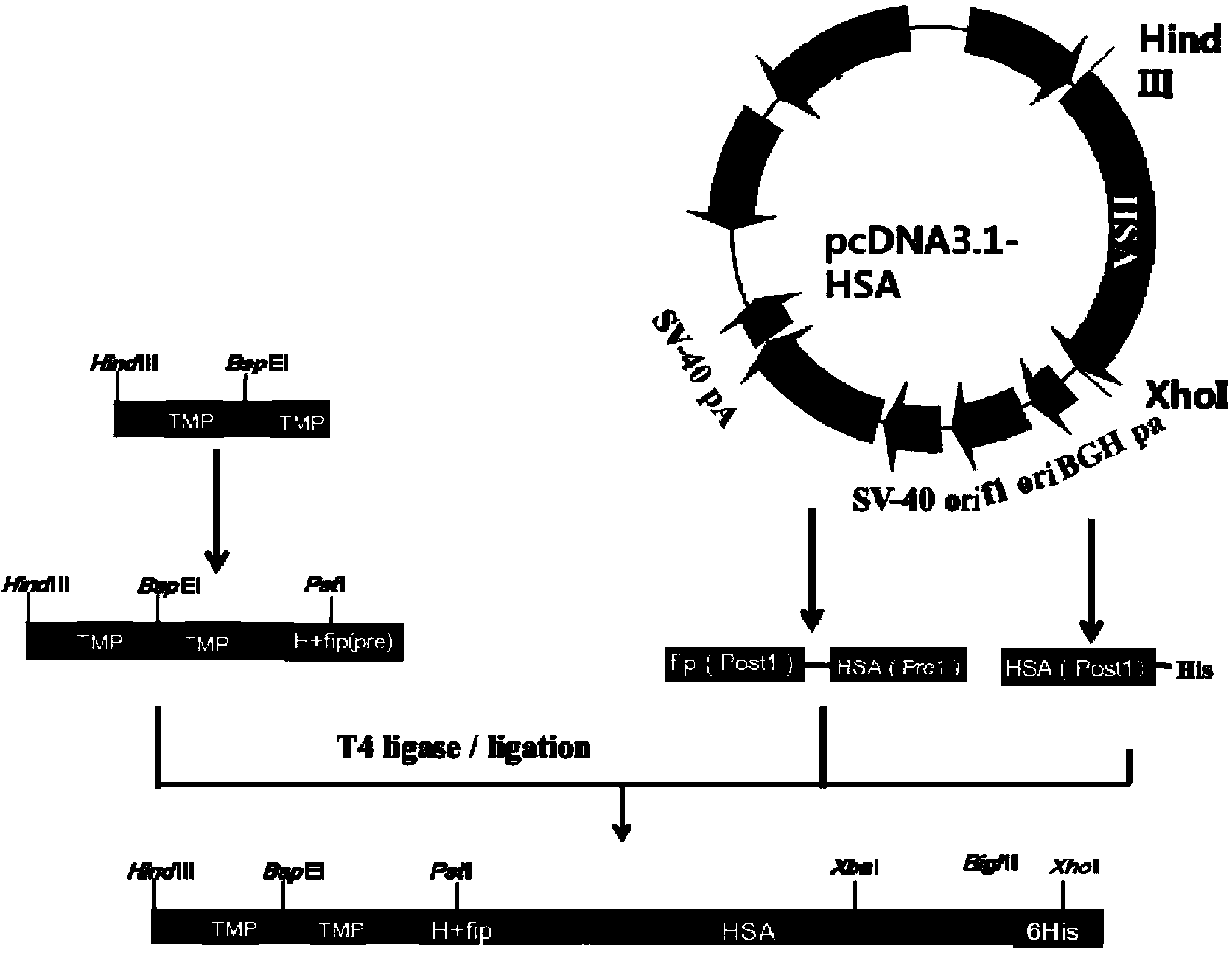
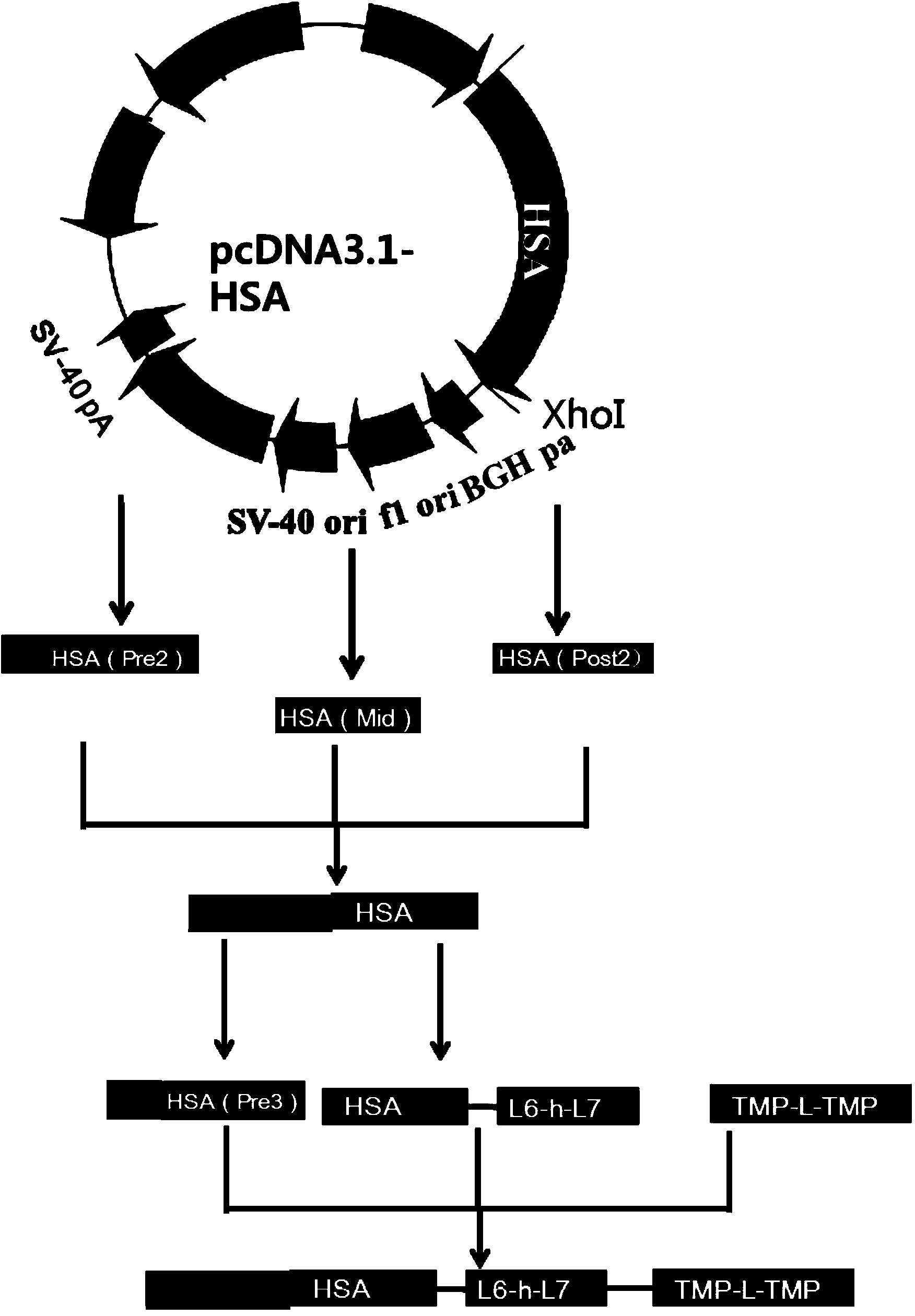
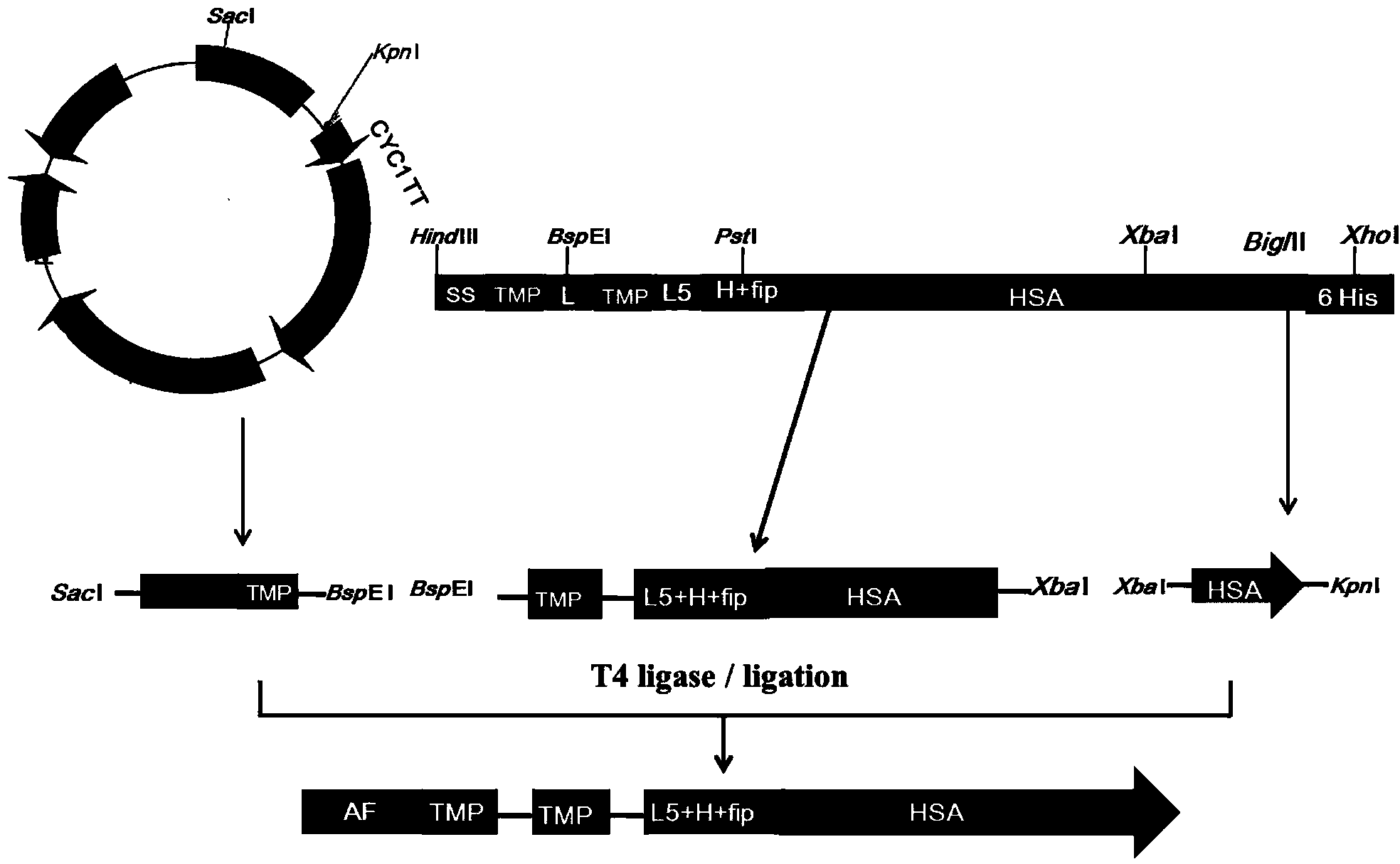
[0075] Example 1 Construction and Screening of Dimerized Thrombopoietin Mimetic Peptide TMP Duplex-Human Serum Albumin Fusion Protein CHO Expression Vector
[0076] One. Construction of the vector comprising TMP-L1-TMP-L5-H-fip-HSA
[0077] Cloning of the fragment of HSA cDNA: HSA cDNA was amplified in two stages from the plasmid pcDNA3.1-fip-HSA (the full gene sequence of HSA was synthesized by Bao Biological Company, and the pcDNA3.1 plasmid was purchased from Invitrogen) by PCR method, respectively. It is fip(Post1)-HSA(Pre1) (fip is a peptide segment selected from the fip domain, HSA is human serum albumin, Pre represents the first half, post represents the second half, and the gene sequence is shown in Seq ID No: 9, The amino acid sequence is shown in Seq ID No: 10) and HSA(Post1)-His (Post is the back end of HSA, His is the tail mark of histidine, and the gene sequence of HSA(Post1)-His is shown in Seq ID No: 11 , the amino acid sequence is shown in Seq ID No: 12). The...
Embodiment 4
[0124] Example Dimerized Thrombopoietin Mimetic Peptide TMP Duplex-Human Serum Albumin Fusion Protein Expression in Mammalian Cell CHO
[0125] A large number of plasmid extraction kits (Qiagen, Shanghai)
[0126] L-glutamine, sodium hypoxanthine and thymine supplementation reagent (HT) were purchased from Invitrogen, USA;
[0127] T75 cell culture flasks were purchased from Denmark NUNC Company;
[0128] 2-L WAVE cell culture bags were purchased from the Medical Group Department of General Electric Company of the United States;
[0129] Chinese hamster ovary suspension cells (CHO-S) were purchased from Invitrogen Corporation of the United States, and the cells were a clone isolated from Chinese hamster ovary subline clone cells (CHO-K1). The CHO-S parental cell line was chosen for cell culture and transfection. CHO-S cells are suitable for serum-free suspension culture using FreeStyle Supplementation Reagent (HT) supplemented with 8mM L-glutamine and 2mM Thymine TM CHO s...
Embodiment 1
[0133] Clones No. 2-8 obtained in Example 1 were also transiently transfected into CHO-S cell lines by the same method, and respectively obtained TMP-L2-TMP-L5-H-fip-HSA sequence, TMP-L2-TMP -L5-H-fip-HSA sequence, TMP-L2-TMP-L5-H-fip-HSA sequence, HSA-L6-H-L7-TMP-L1-TMP sequence, HSA-L6-H-L7-TMP- L2-TMP sequence, HSA-L6-H-L7-TMP-L3-TMP sequence, HSA-L6-H-L7-TMP-L4-TMP sequence encoding target product supernatant cells transient expression.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com