Stem cell in-vitro multiplication culture system and method
A culture system and in vitro amplification technology, applied in the field of biomedicine, can solve the problems of donor injury, failure to find a single umbilical cord blood, and limited number of stem cells, so as to save storage space, reduce physical storage capacity, and reduce possible damage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation and Purification of Umbilical Cord Blood CD34+ Cells
[0033] After obtaining the informed consent of the puerpera, obstetricians and medical staff aseptically collected cord blood from healthy full-term newborns, transported the blood samples to a 10,000-level aseptic laboratory, and performed preparation operations in a 100-level aseptic biological safety cabinet. The operator draws about 10 mL of umbilical cord blood from the umbilical cord blood sample, measures the number of white blood cells in the whole blood, and adds 60 g / L of hydroxyethyl starch to the remaining blood sample, umbilical cord blood: hydroxyethyl starch = 1:4 (v / v), Gently mix to promote erythrocyte sedimentation, then place the blood sample in a large-capacity low-temperature centrifuge and centrifuge at 630rpm for 6.5min at 8-12°C, then manually press the slurry to separate the upper layer of plasma from the centrifuged blood sample , white blood cells (including hematopoi...
Embodiment 2
[0034] Example 2 In vitro expansion and culture of hematopoietic stem cells
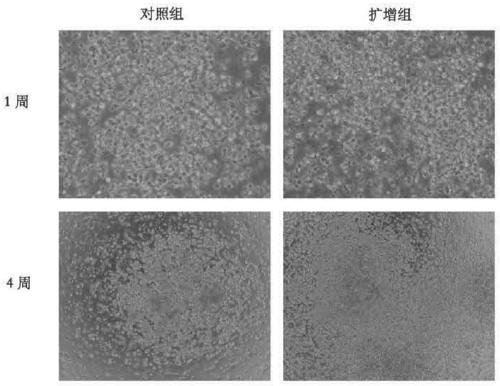
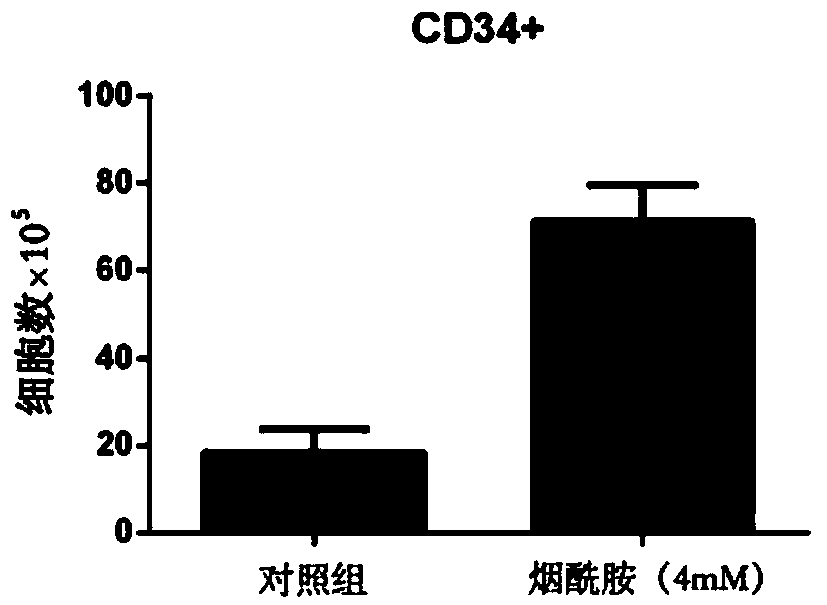
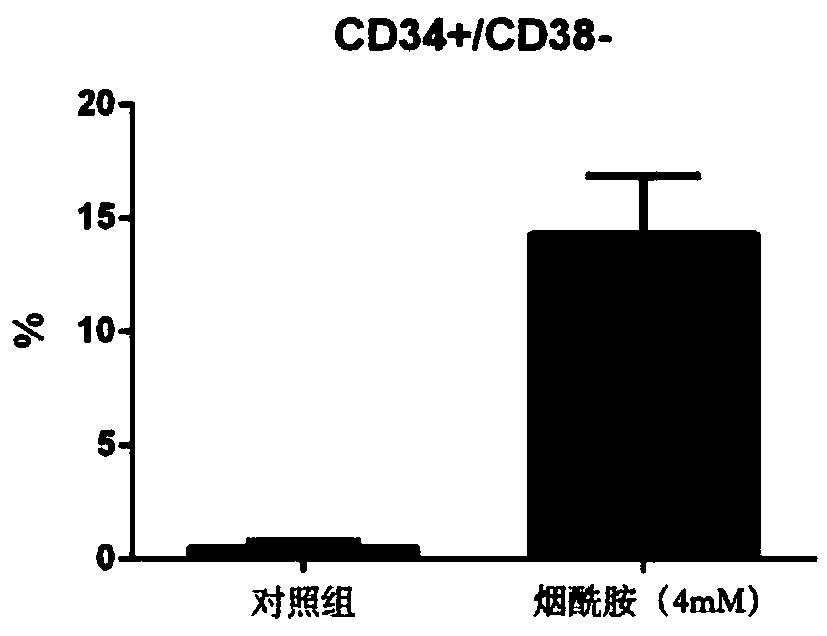
[0035] The CD34+ cells prepared and purified according to the method described in Example 1 were inoculated on a 12-well plate (Corning Costar Company, the United States), and a control group and an expansion group were set up, and the cell inoculation density was 1 × 10 4 / mL.
[0036] The expansion group cells were added with expansion medium, 2mL / well, and the expansion medium contained: serum-free medium (StemCellTechnologies, Vancouver, BC) as the base medium, 10% (v / v) fetal bovine serum (Gibco, U.S. ), 4.5mM Nicotinamide, 20ng / mL TPO, 50ng / mL IL-6, 60ng / mL FLT-3 Ligand, 40ng / mL SCF, 20ng / mL IL-3, 30ng / mL G-CSF, and 15ng / mL EPO. The culture medium of the control group was the expansion medium without nicotinamide, and the dosage was 2 mL / well. Place cells at 37°C, 5% CO 2 , and a sterile incubator with saturated humidity, and the cells were fully replaced every 5 days. When the cell densit...
Embodiment 3
[0038] Example 3 Determination of hematopoietic stem cell colony-forming ability
[0039] The hematopoietic stem cells prepared and purified according to the method described in Example 1 were diluted with 5% glacial acetic acid, and the cells were counted by trypan blue staining. The culture medium adjusted the cell suspension concentration to 1×10 4 / mL, mix thoroughly, then, draw the cell suspension with a sterile syringe, add 2mL / well into a six-well culture plate (Corning Costar, USA), repeat three holes; gently shake the culture plate to make the cells evenly fill the whole At the bottom of the culture plate, mark the sample number and inoculation time with a marker pen above the culture well of the culture plate, draw a "cross" on the bottom of the culture well to divide the bottom into 4 areas, so that the colonies can be counted later, and finally, culture Plates were placed at 37°C, 5% CO 2 , saturated humidity in a sterile cell incubator, and then observe and coun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com