Recombinant targeted infuse protein for promoting thrombocytopoiesis and its preparation method
A fusion protein and platelet technology, which is applied to medical preparations containing active ingredients, drug combinations, peptide/protein components, etc., can solve the problems of increasing the cost of use and increasing the amount of use, and achieve enhanced effects, reduced dosage, The effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Cloning of hGH
[0067] The cDNA sequence of hGH gene was obtained by PCR from the fetal liver cDNA library, and the PCR upstream and downstream primers used for amplification were GH1 and GH2, respectively. The base sequences of GH1 and GH2 are as follows:
[0068] GH-1: 5′-ATG TTC CCA ACT ATT CCA CTG-3′
[0069] GH-2: 5′-TTA GAA GCC ACA CGA CCC TTC-3′
[0070] The PCR reaction system is 100ul, which contains 100pmol of primers GH-1 and GH-2, 20nmol of dNTP, 1ul of fetal liver cDNA library, 10ul of 10×PCR reaction buffer, and 5U of high-fidelity Pyrobest DNA polymerase. The reaction conditions of PCR were as follows: the first stage of pre-denaturation at 95°C for 5 minutes; the second stage of denaturation at 94°C for 1 minute, annealing at 55°C for 1 minute, extension at 72°C for 1 minute, and 32 cycles; the third stage of extension at 72°C for 5 minutes, 10°C for 10 minutes. The obtained PCR product is detected by 1% agarose electrophoresis, and an el...
Embodiment 2
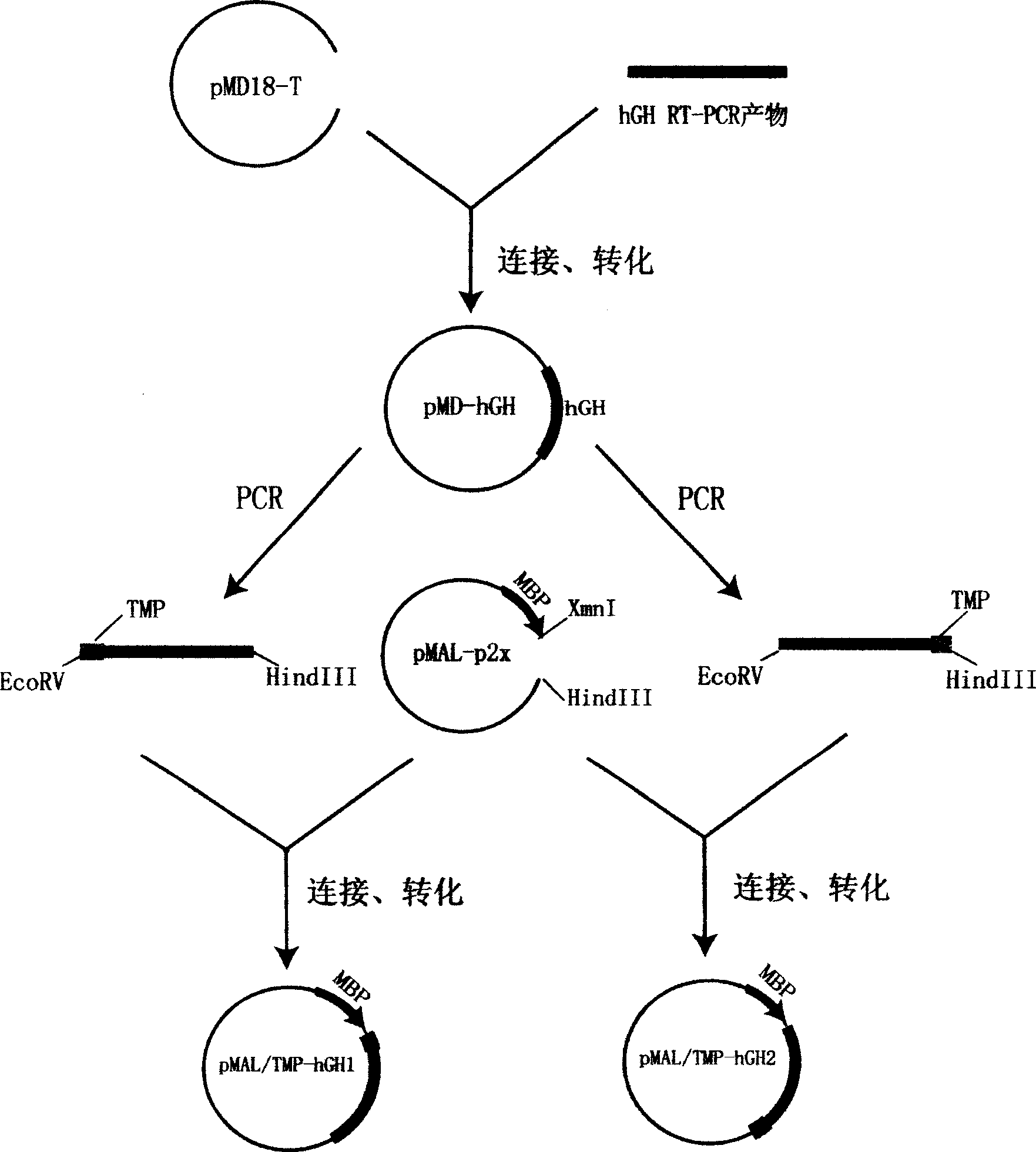
[0071] Example 2: Construction and Identification of TMP and hGH Fusion Protein Expression Plasmid
[0072] In order to enable TMP to form a fusion protein with hGH, the following two PCR primers were first designed and synthesized:
[0073] MH-1: 5′-ACT GAT ATC GAA GGT CCG ACT CTG CGT CAG TGG CTG GCT GCACGT GCT GGT TCT GGT TCT GGT TTC CCA ACT ATT CCA CTG AG-3′
[0074] MH-2: 5′-AGT AAG CTT TTA GAA GCC ACA CGA CCC TTC-3′
[0075] In MH-1, the underlined part is the recognition site of the endonuclease EcoRV, the bold part is the nucleotide sequence encoding TMP (SEQ ID NO1 of the sequence table), and the italic part is the encoding bridge peptide Gly-Ser-Gly-Ser - the nucleotide sequence of Gly. The underlined part in MH-2 is the recognition site of endonuclease Hind III.
[0076] Such as figure 1 As shown, the pMD-hGH plasmid DNA was used as a template to amplify the hGH gene by PCR, and the PCR amplification conditions were the same as above. The obtained PCR produ...
Embodiment 3
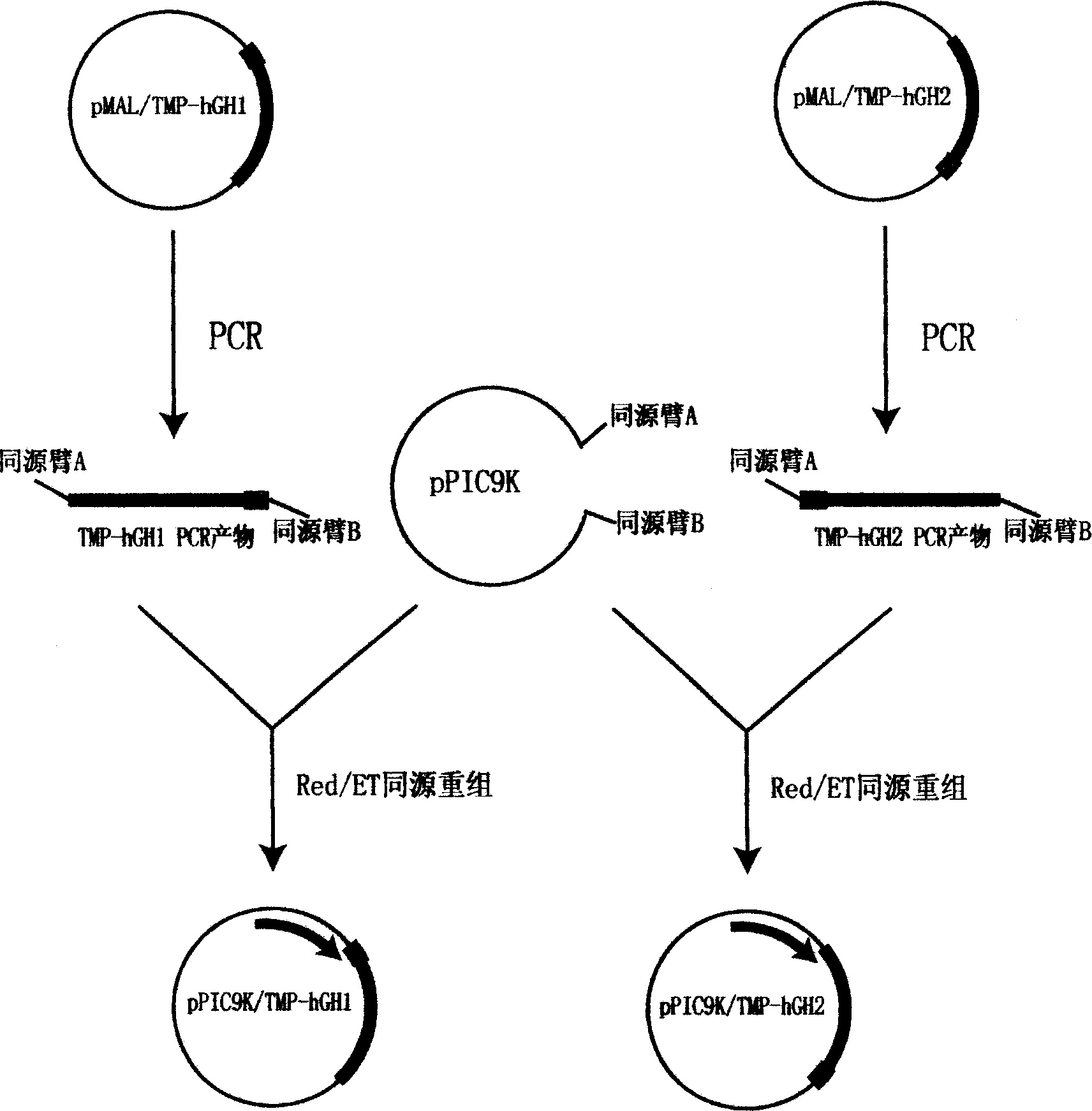
[0093] Embodiment 3: the fermentation of engineered yeast and the purification of TMP-hGH fusion protein
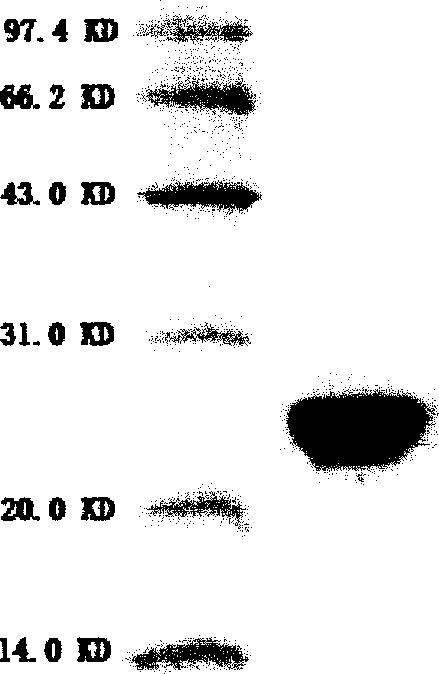
[0094] Inoculate pPIC9K / TMP-hGH engineered yeast strains containing pPIC9K / TMP-hGH stored at -70°C on a YPD plate for activation, pick a single colony with good appearance and inoculate it in BMGY medium, and culture in a constant temperature shaker at 280rpm at 28°C-30°C for 16-18 hours to OD 600 ≈4, the bacterial liquid is used as the seed liquid. Then the seed solution was inoculated in a 15L fermenter (B. Braun company, Germany) equipped with 2.5L BMMY medium for fermentation. The fermentation temperature is controlled at 30°C, the dissolved oxygen is greater than 20% saturation, the pH value is around 5.0, and cultured to OD 600 When ≈150, methanol was added for induction, so that the final concentration of methanol was always maintained at about 1%. After induction culture for 72 hours, the supernatant was obtained by centrifugation, 20% ammonium sulfate was adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com