Device and method for in vitro high-density cultivation of erythrocyte
A high-density culture and red blood cell technology, applied in the field of regenerative medicine, can solve the problems of large volume, multiple immunity, and the decline of blood donation population, and achieve the effect of reducing volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 A device for in vitro high-density culture of red blood cells
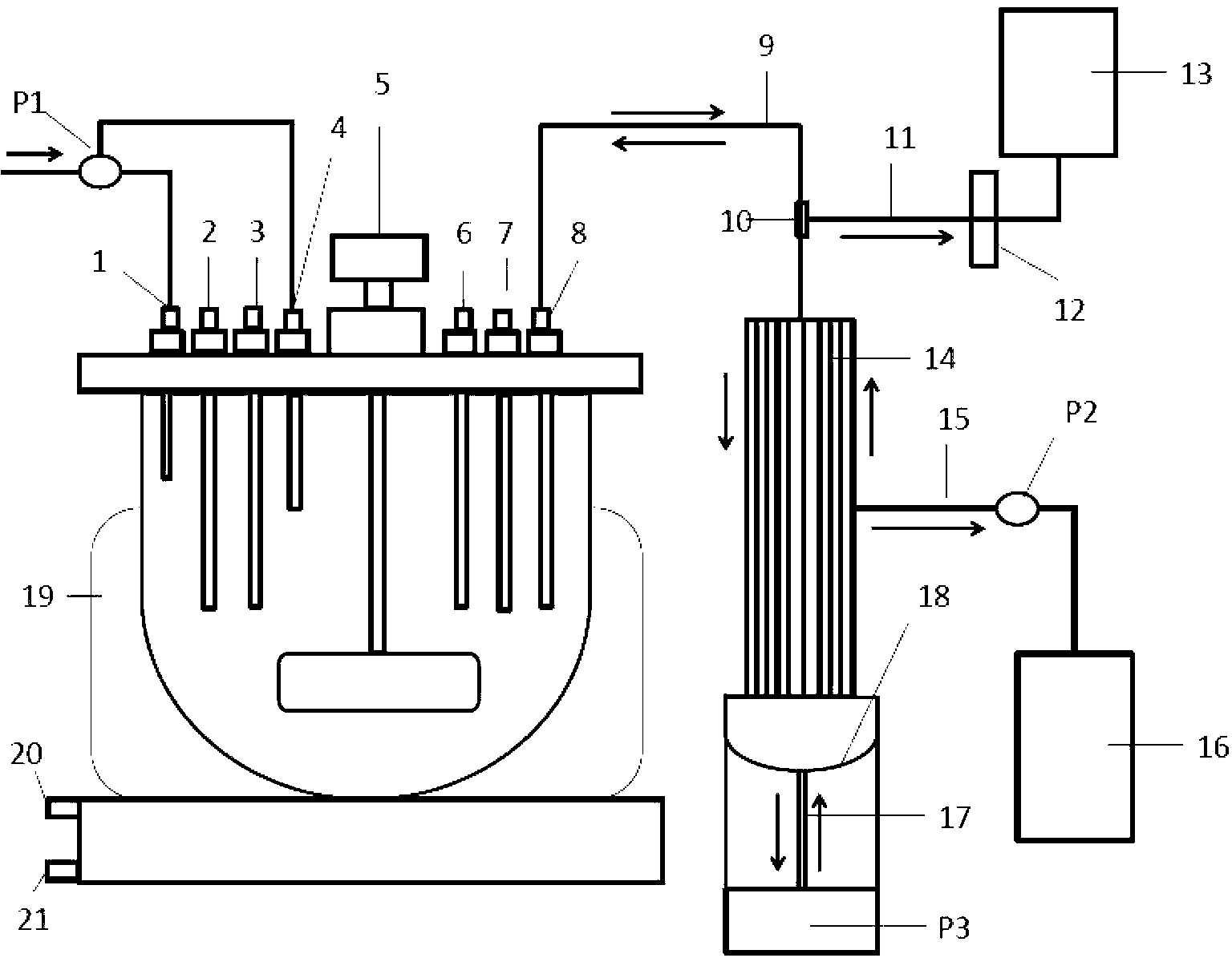
[0052] Includes stirred bioreactors and hollow fiber cell retention devices such as figure 1 As shown, wherein, the structure of the hollow fiber cell trapping device is: a cylindrical sleeve 14 composed of 4 to 10 hollow fiber tubes parallel to each other, and the bottom of the cylindrical sleeve 14 is sequentially provided with an elastic valve 18, a piston 17 and a Vacuum pump P3, the middle part of the cylindrical casing 14 is provided with a waste liquid pipe 15, the end of the waste liquid pipe 15 is provided with a waste liquid bag 16, and the waste liquid pipe 16 is provided with an osmotic pump P2, and the upper part of the cylindrical casing 14 passes through the connecting pipe 9 and The stirring bioreactor is connected, the connecting pipe 9 is provided with a leukocyte removal filter 10, the connecting pipe 9 is connected with a flexible pipe 11, the end of the flexible pipe 11 is pro...
Embodiment 2
[0056] Example 2 Using the device in Example 1 to culture red blood cells at high density in vitro (taking hematopoietic stem cells derived from umbilical cord blood as an example)
[0057] Proceed as follows:
[0058] (1) Isolation of cord blood mononuclear cells (MNC):
[0059] (1) Put 50ml of umbilical cord blood sample in a 50ml centrifuge tube and centrifuge at 2000rpm for 10min;
[0060] The umbilical cord blood sample can be purchased or collected by yourself. When collecting by yourself, collect according to the standard for umbilical cord blood sample collection. Full-term or premature delivery, infectious diseases and various family genetic diseases are excluded, and the sample collection is strictly aseptic. ; Use compound citrate blood protection solution for anticoagulation; the average volume of cord blood is 50-100ml, and the specimens are separated within 3 hours after collection.
[0061] (2) Transfer the upper layer of plasma to another 50ml centrifuge tube...
Embodiment 3
[0079] Example 3 Comparison of different sources of hematopoietic stem cells induced to generate erythrocytes in vitro
[0080] (1) Hematopoietic stem cells from three different sources: bone marrow (M0), umbilical cord blood (SC) and fetal liver (FF) were used to induce red blood cells in vitro, and the culture process was the same as in Example 2; Compared, the results are as follows:
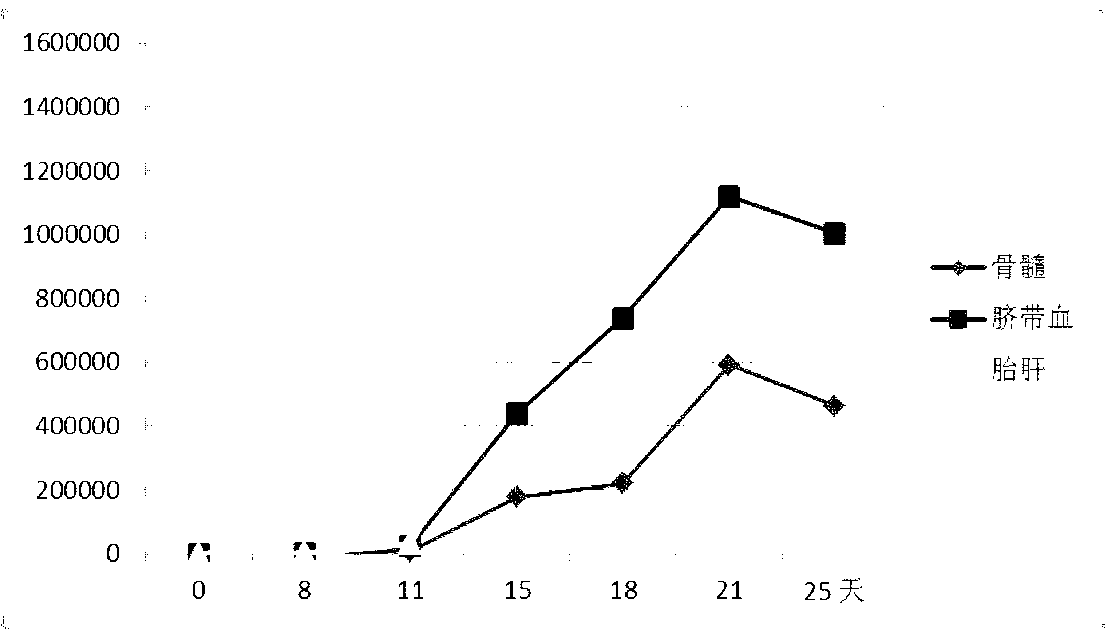
[0081] The value-added curve compares with figure 2 shown by figure 2 It shows that the hematopoietic stem cells derived from fetal liver have the strongest proliferative ability in vitro, the hematopoietic stem cells derived from umbilical cord blood are second, and the proliferative ability of hematopoietic stem cells derived from bone marrow is the weakest.
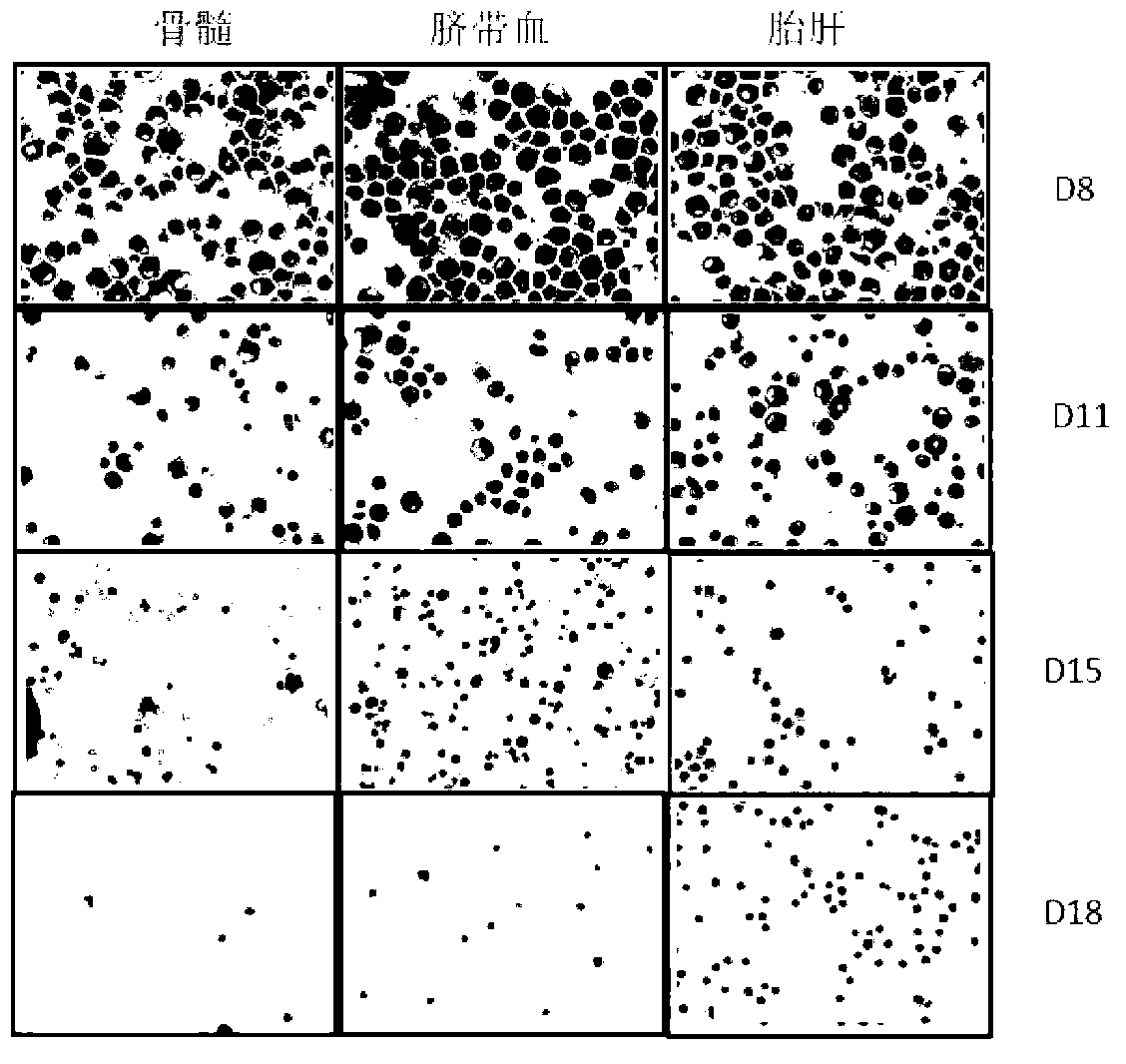
[0082] Red blood cell maturity (morphology) compared to image 3 shown by image 3 It shows that the denucleation rate of hematopoietic stem cells from different sources of hematopoietic stem cells in vitro is that bone marrow-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com