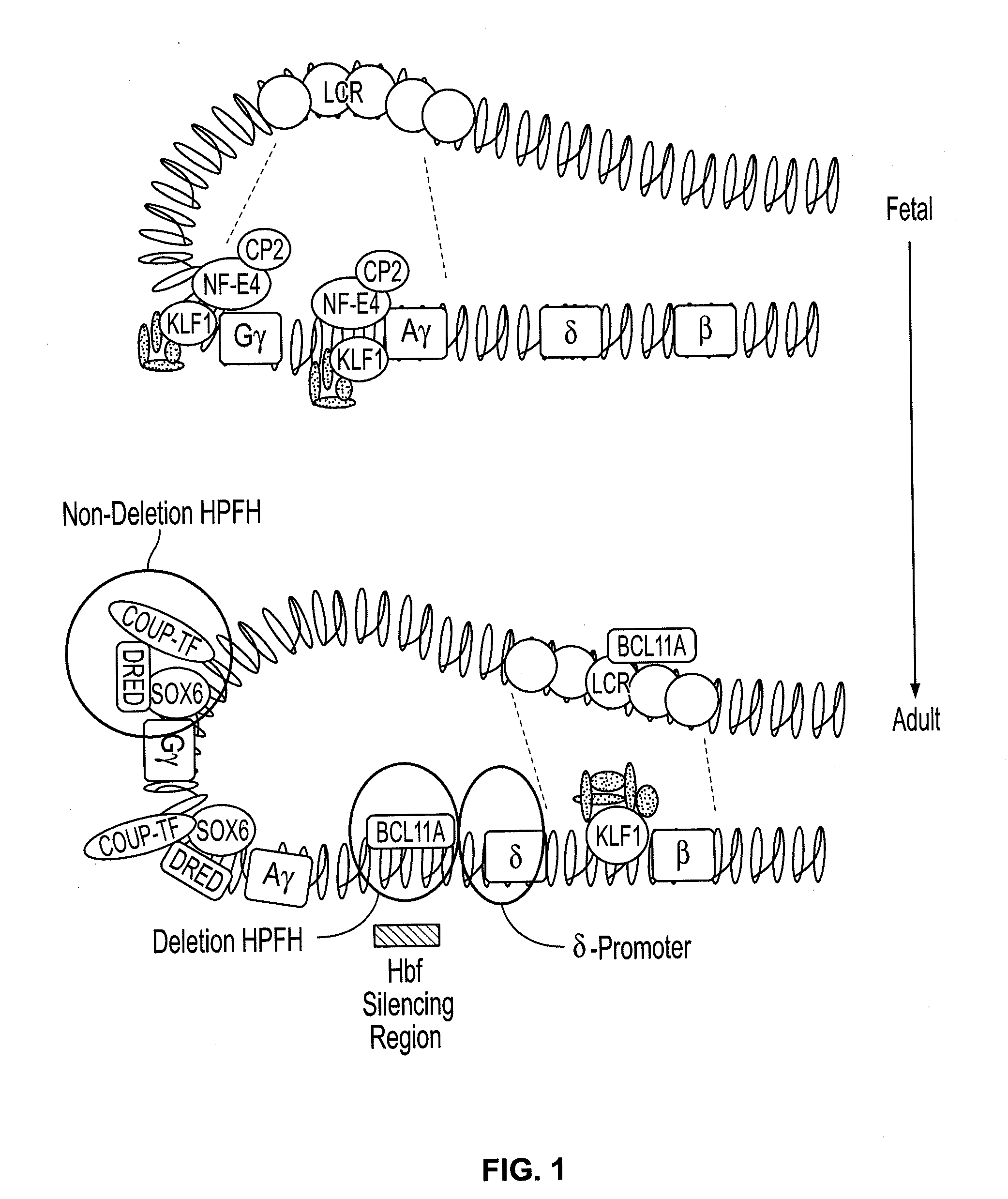
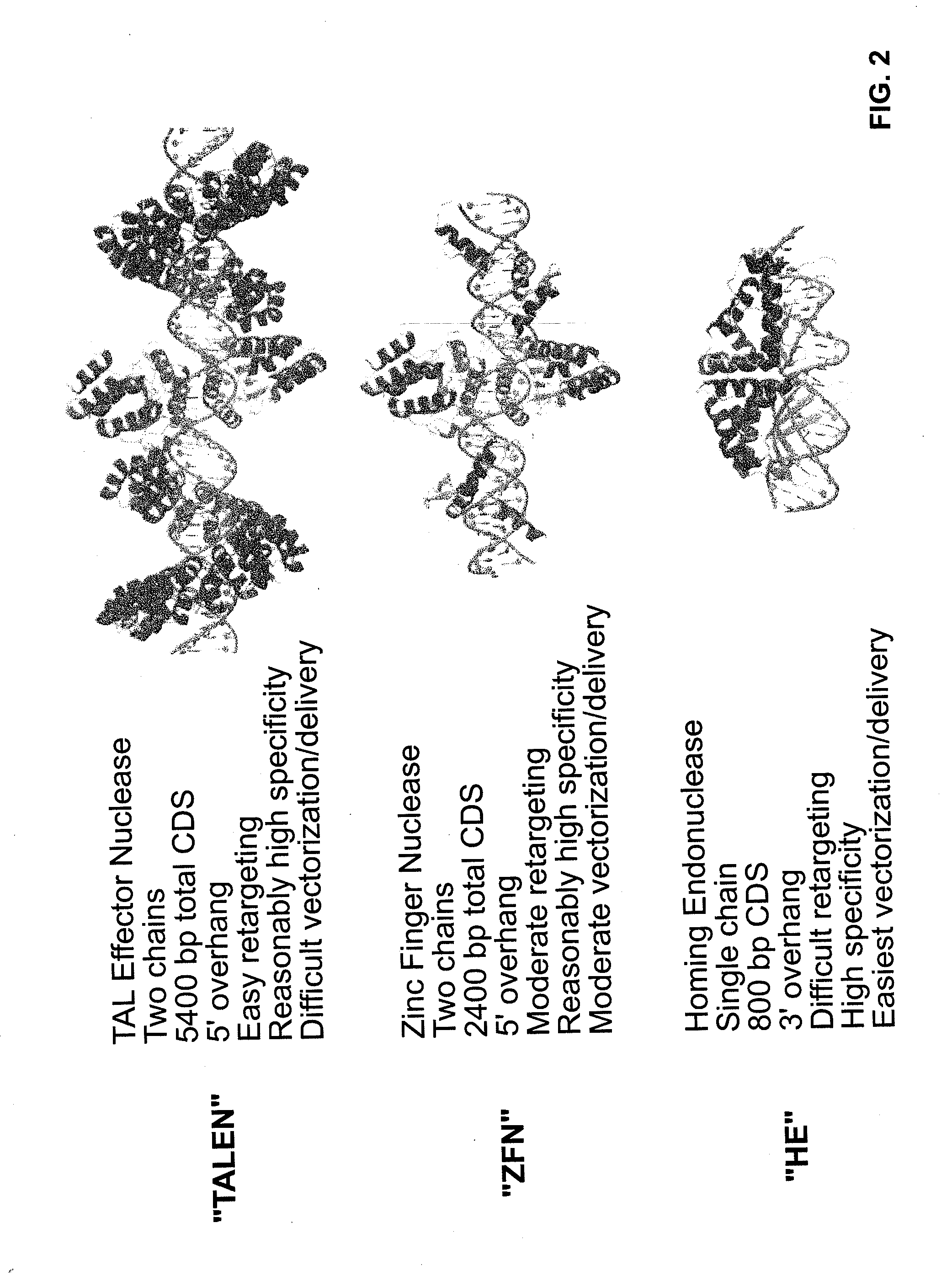
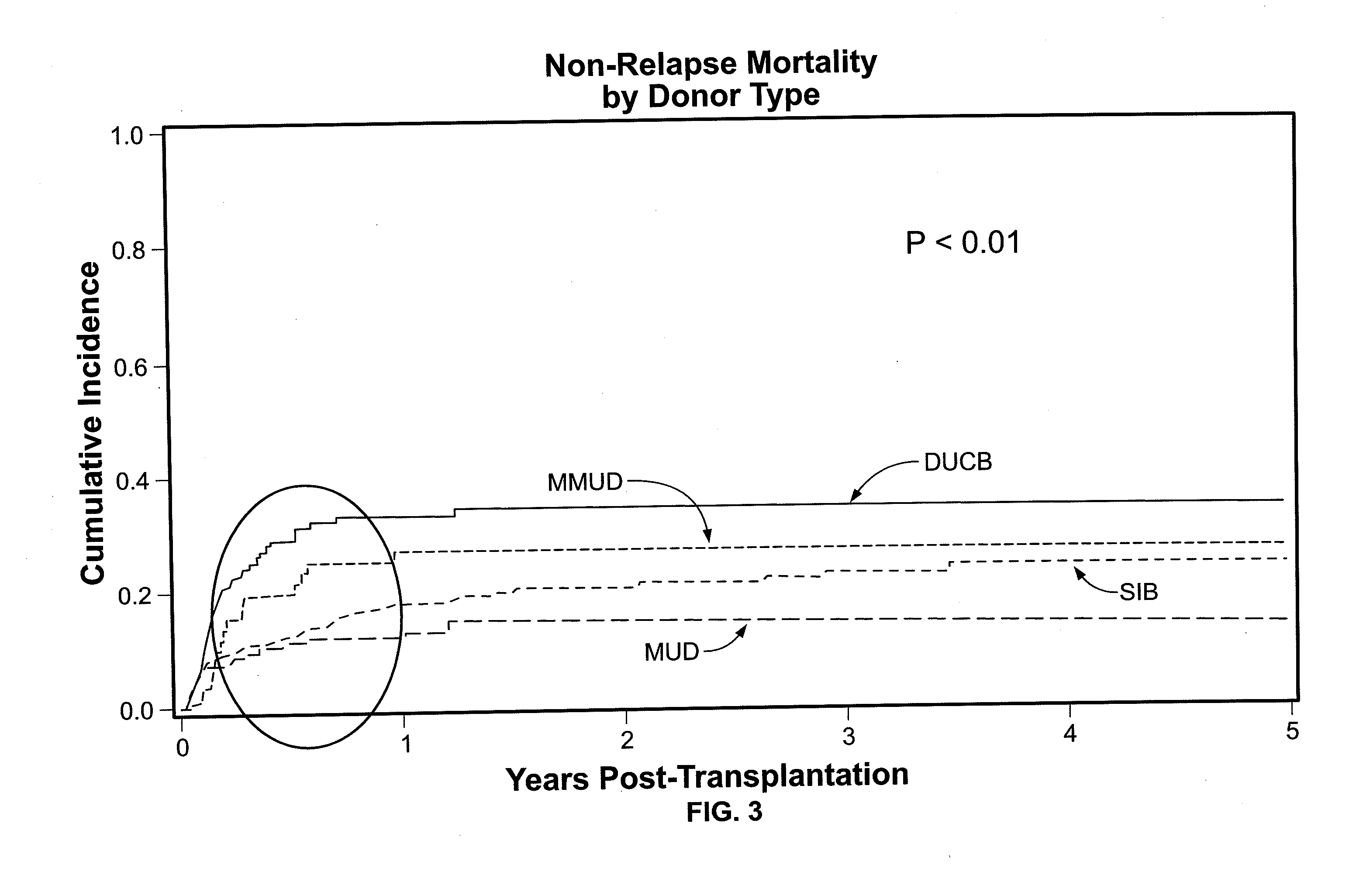
Compositions and methods for the treatment of hemoglobinopathies
a technology for hemoglobinopathies and compositions, applied in the field of genetic diseases, can solve the problems of unpaired -like chains, significant health burden worldwide, and unpaired -like chains, and achieve the effects of enhancing the survival and proliferation of hematopoietic stem/progenitor, enhancing the expansion of long-term repopulating cells, and efficient engrafting of corrected cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Selection of Bcl11a Gene Targeting Homing Endonucleases Based on I-HjeMI, I-CpaMI, and I-OnuI Using In Vitro Compartmentalization (IVC)
[0157]The open reading frame (ORF) of a parental LAGLIDADG homing endonuclease (LHE), I-HjeMI (FIG. 14; SEQ ID NO: 28; Jacoby et al., Nucl. Acids Res. 40(11):4954-4964 (2012), Taylor et al., Nucl. Acids Res. 40(Web Server issue): W110-6 (2012)), codon optimized for expression in E. coli, was cloned between the NcoI and NotI restriction sites of pET21-a(+) (FIG. 11; EMD Millipore (Novagen) division of Merck KGaA. To introduce site-directed saturation mutagenesis into the ORF of I-HjeMI, DNA fragments containing its partial ORF with approximately 20 base pairs of a region overlapped with flanking fragments on both sides were PCR-amplified using primers that contained degenerate codon 5′-NNK-3′ (coding all 20 amino acids). Amino-acid residues mutated using such PCR primers are shown in Table 2. PCR products were purified by extraction from an agarose ge...
example 2
Optimization of Activity of BCL11A Gene-Targeting I-HjeMI Variants Using Two-Plasmid Gene Elimination Cleavage Assay in Bacteria
[0163]The activity of I-HjeMI variants obtained in selection using IVC display selections (disclosed in Example 1, above) was optimized using a two-plasmid selection system in bacterial cells according to the methodology of Doyon et al., J. Am. Chem. Soc. 128(7):2477-2484 (2006). The ORF of the endonuclease genes was inserted between NcoI and NotI sites of the pENDO (FIG. 12, Doyon et al., J. Am. Chem. Soc. 128(7):2477-2484 (2006) expression plasmid. NovaXGF (EMD Millipore (Novagen)) competent cells harboring the pCcdB reporter plasmid (FIG. 31, Doyon et al., J. Am. Chem. Soc. 128(7):2477-2484 (2006); Takeuchi et al., Nucl. Acids Res. 37(3)877-8890 (2009); and Takeuchi et al., Proc. Natl. Acad Sci. U.S.A. 10.1073 / pnas.1107719108 (2011)) containing 4 copies of the BCL11A gene target were transformed with a pool of the pEndo plasmid encoding I-HjeMI variants....
example 3
Activity of BCL11A Gene-Targeting Endonucleases Tested in a Two-Plasmid Cleavage Assay
[0165]Activity of an exemplary BCL11A gene-targeting endonuclease (BCL11Ahje; FIG. 17, SEQ ID NO: 31), its catalytically inactive variant (BCL11Ahje D18N), and its parental LHE I-HjeMI (FIG. 14, SEQ ID NO: 28) was measured in bacterial cells that harbor the pCcdB reporter plasmid (Doyon et al., J. Am. Chem. Soc. 128(7):2477-2484 (2006)) containing 4 copies of either the target site for I-HjeMI (I-HjeMI target) or the BCL11A gene target (TCCAAGTGATGTCTCGGTGGTG (SEQ ID NO: 39; underlined nucleotides differ from those in the target site for the parental LHE I-HjeMI). The pCcdB reporter plasmid encodes “control of cell death B” (“ccdB”, a toxic protein in bacteria, which is inducible by an addition of IPTG). Cleavage of the target sites in the reporter plasmid leads to RecBCD-mediated degradation of the reporter plasmid and corresponding cell survival on the selective medium containing IPTG. The surviv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com