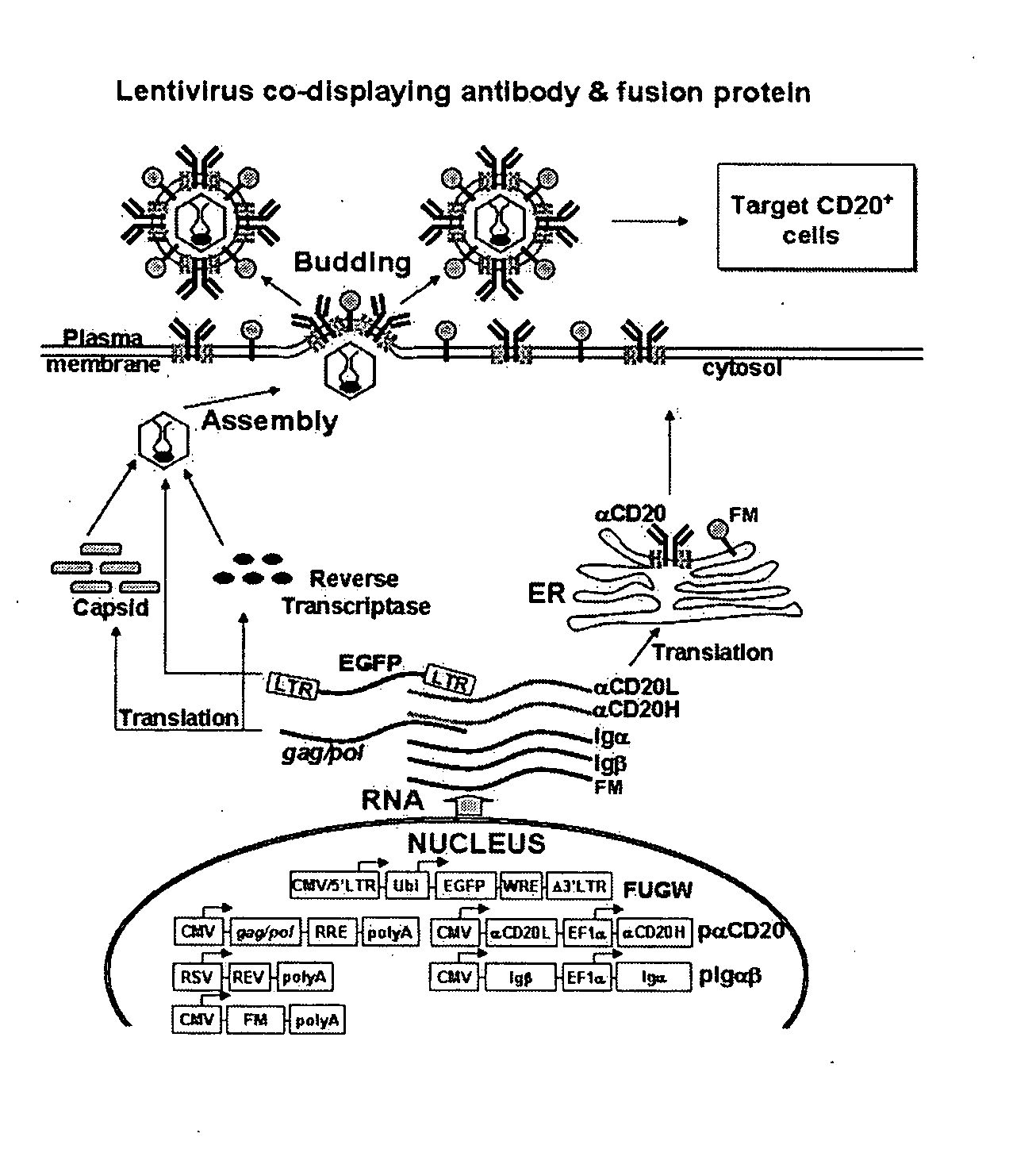
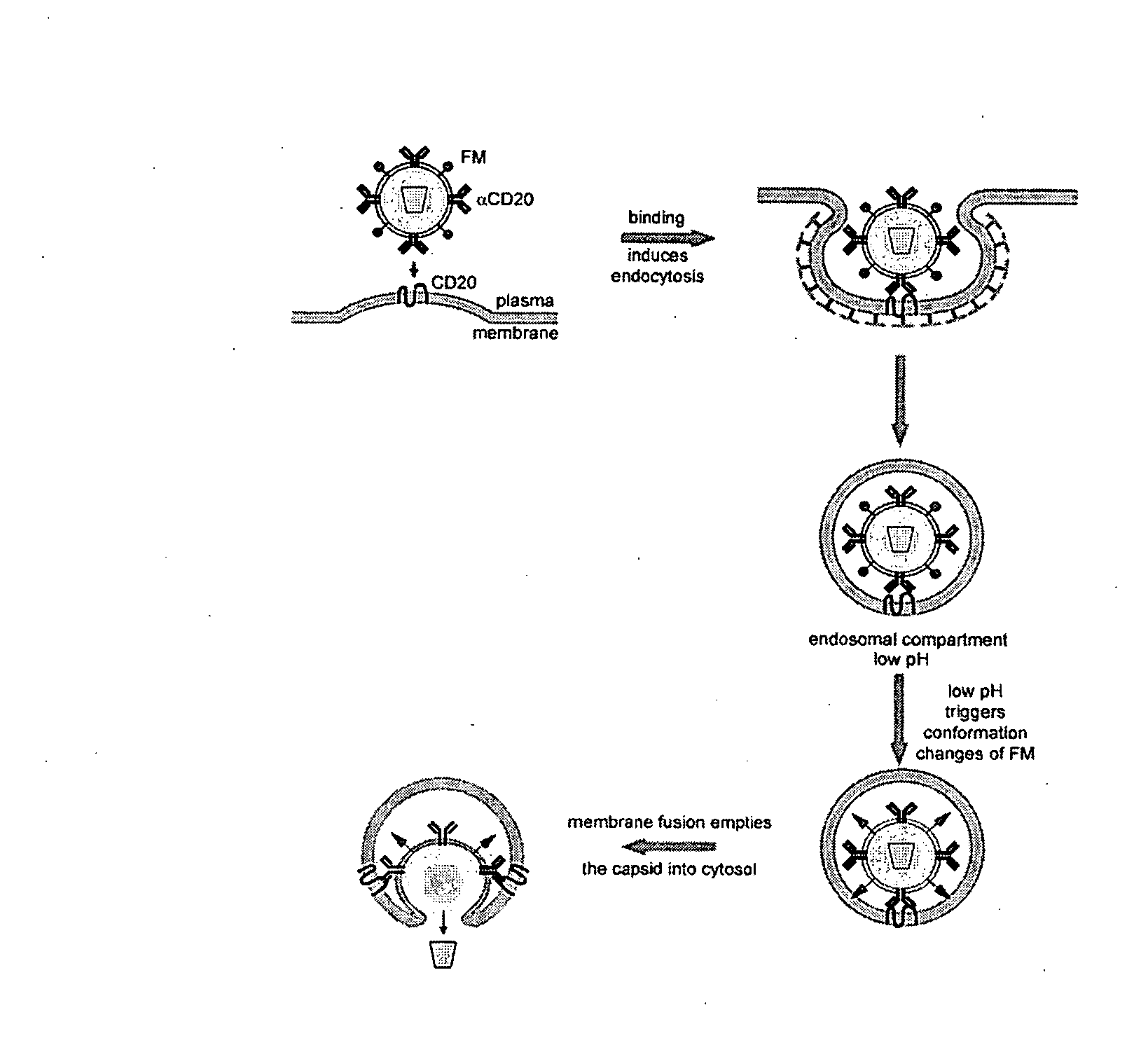
Method of targeted gene delivery using viral vectors
a viral vector and gene technology, applied in the field of targeted gene delivery, can solve the problems of low viral titers, difficult to reconstitute fusion function, and difficult to target viruses to particular cell types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Transduction of CD20-expressing Target cells and 293T Cells Utilizing the Retroviral Vector FUGW / αCD20+HAmu and FUGW / 60 CD20+SINmu
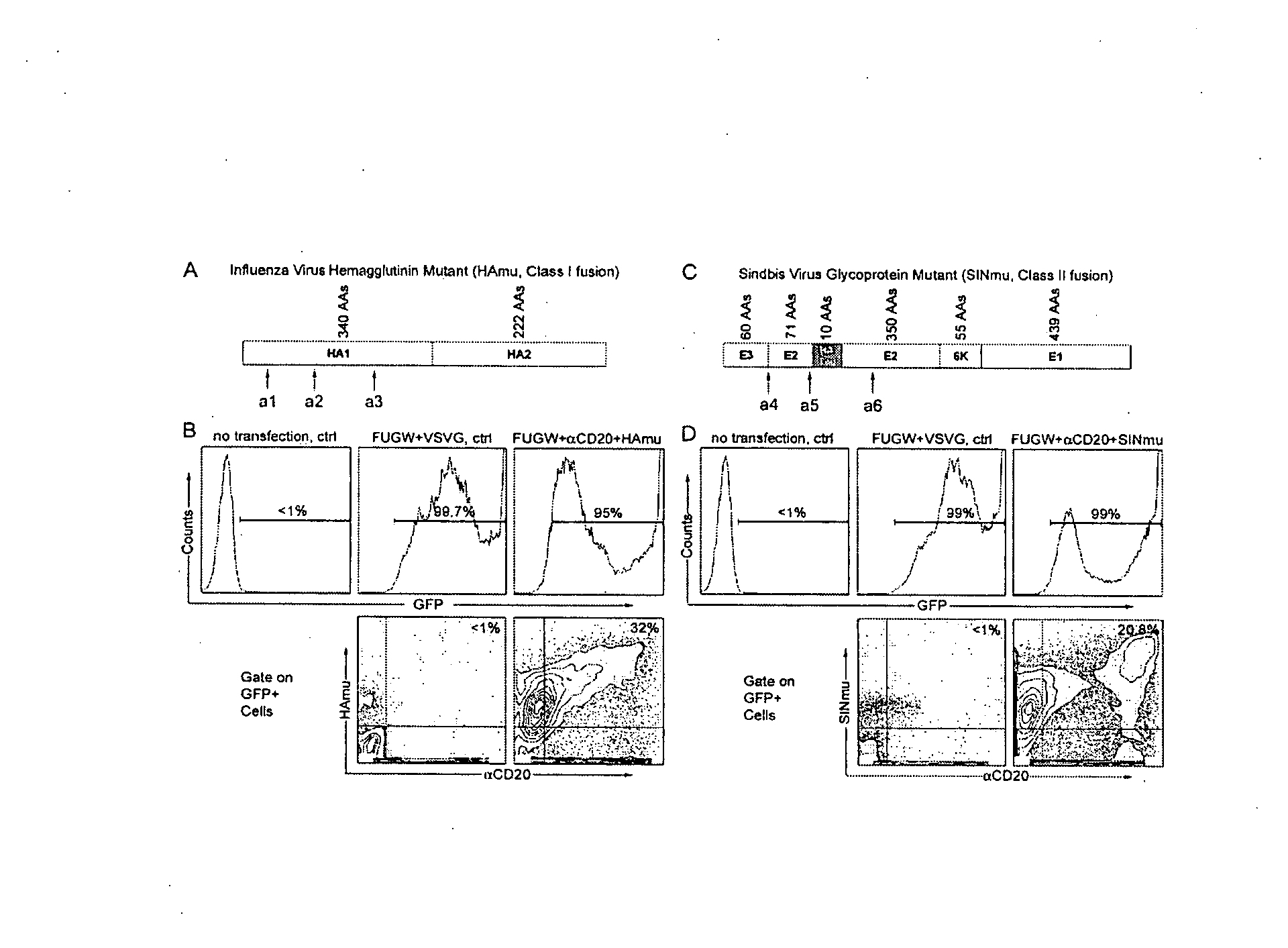
[0248] Next, the efficacy of a αCD20-bearing virus in transferring genes into cells expressing CD20 in a cell-specific manner was tested. GFP expression was used to measure the transduction efficiency. The supernatants containing virus bearing various surface proteins were incubated with CD20-expressing target cells and 293T cells served as a control. Four days post-transduction, the efficiency of targeting was analyzed by FACS. FIG. 5A (rightmost panel) shows that FUGW / αCD20+HAmu viral particles could specifically transduce 16% of 293T / CD20 cells. Panels to the left show that transduction required the presence on the virions of HAmu, but there was some background transduction with virions lacking αCD20, likely due to residual weak binding of HAmu to its ligand, sialic acid. The titer for FUGW / αCD20+HAmu (fresh viral supernatant, no concentration) was es...
example 3
Transduction of Primary Human B-Lymphoid Cells Using the Retroviral Vector FUGW / αCD20+SINmu
[0254] Having established the ability of the system to mediate CD20-specific transduction of artificially created cell lines, the specific transduction of primary human B-lymphoid cells, cells that naturally carry the CD20 antigen, was investigated. Fresh, unfractionated human peripheral blood mononuclear cells (PBMCs) were transduced with FUGW / αCD20+SINmu and then stimulated with lipopolysaccharide (LPS) to expand the B cell population. Four days later, the cells were stained for CD19 (a B cell marker), CD20 and GFP expression (FIG. 6A). Over 35% of cells were CD20+ B cells under the described culture condition. The majority of them were GFP+. On the contrary, virtually no GFP+ cells were detected among CD20− non-B cells, confirming that the transduction was strictly dependent on CD20 expression. In another control experiment, fresh PBMCs were transduced with FUGW / αCD20+SINmu followed by sti...
example 4
Demonstration of Transduction Utilizing Lentiviral Vectors CCMV / αCD20+SINmu and CPGK / αCD20+SINmu
[0255] To demonstrate that the targeting method is not limited to the lentiviral vector FUGW, two additional lentiviral vectors with different promoter configurations were evaluated. Kohn et al. have incorporated the immunoglobulin heavy chain enhancer (Eμ) with associated matrix attachment regions into lentivectors carrying either the human cytomegalovirus (CMV) promoter (CCMV) or the murine phosphoglycerate kinase promoter (CPGK) (C. Lutzko et al. J Virol. 77, 7341-51 (2003)). These two lentiviral vectors were then adapted into the system and recombinant lentiviruses CCMV / αCD20+SINmu and CPGK / αCD20+SINmu were prepared. Transduction of PBMC-derived B cells with these viral supernatants exhibited results similar to those observed previously with FUGW (FIG. 6A). Stable integration of the GFP-transgene was detected by genomic PCR amplification (FIG. 6B).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com