Hyperbranched polythioether polyamine, and preparation method and use thereof
A polysulfide and application technology, which is applied in the field of hyperbranched polysulfide polyamine and its preparation, can solve the problems of high equipment requirements and strict reaction conditions, and achieves the effects of low equipment requirements, simple preparation method and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of preparation method of hyperbranched polysulfide polyamine proposed by the present invention comprises the following steps;
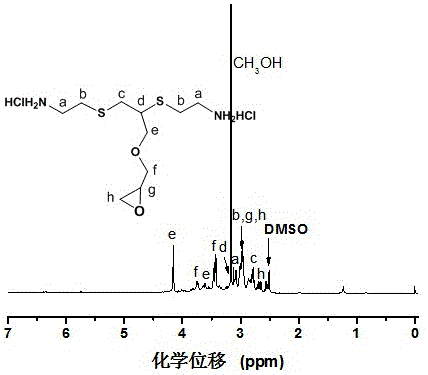
[0028] S1: Put 1.12 grams (0.01 moles) of propynyl glycidyl ether and 2.27 grams (0.02 moles) of cysteamine hydrochloride into the reactor, and add 128 milligrams (0.5 millimoles) of benzoin dimethyl Ether and 30.5 grams of methanol, and under the irradiation of a 200W high-pressure mercury lamp, reacted at room temperature for 4 hours to obtain the α-epoxy-ω-amine hydrochloride solution. The chemical structure of α-epoxy-ω-amine hydrochloride was characterized by NMR, the results are as follows figure 1 shown.
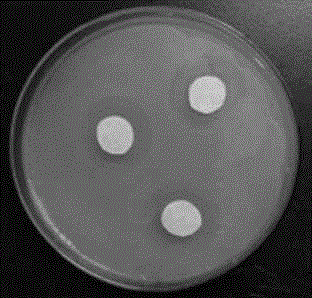
[0029] S2: Add 10.13 g (0.10 moles) of triethylamine to the above-mentioned concentrated product, turn on the stirring device, and raise the temperature to the reflux temperature of the solvent for 24 hours to obtain hyperbranched polythioether polyamine hydrochloride. Its antibacterial and bactericidal properties are shown in...
Embodiment 2
[0032] A kind of preparation method of hyperbranched polysulfide polyamine proposed by the present invention comprises the following steps;
[0033] S1: Put 1.12 g (0.01 mol) of propynyl glycidyl ether and 1.70 (0.015 mol) of cysteamine hydrochloride into the reactor, and add 0.05 mmol of 2-hydroxymethylphenylpropane-1 -ketone and 42.3 grams of methanol-ethanol mixed solvent (volume ratio 7:3), and under the irradiation of a 100W high-pressure mercury lamp, reacted in an ice-water bath for 12 hours to obtain an α-epoxy group-ω-amine hydrochloride intermediate The solution;
[0034] S2: Add 1.52 g (0.015 mol) of triethylamine to the above product, turn on the stirring device, raise the temperature to 65° C., and react for 60 hours. Obtain hyperbranched polythioether polyamine hydrochloride solution;
[0035] S3: Add 2.70 g (0.015 mol) of sodium methoxide-methanol solution (concentration: 30%) to the above hyperbranched polythioether polyamine hydrochloride solution, and conti...
Embodiment 3
[0037] A kind of preparation method of hyperbranched polysulfide polyamine proposed by the present invention comprises the following steps;
[0038] S1: 1.12 g (0.01 mol) of propynyl glycidyl ether and 1.70 g (0.015 mol) of cysteamine hydrochloride were added to the reactor, and 0.15 mmol of 2-methyl-1-[4- Methylthiophenyl]-2-morpholino-1-propanone and 33.84 grams of methanol-water solution (volume ratio 4:1), and at 12000μW / cm 2 Under the irradiation of a special ultraviolet lamp, react at room temperature for 6 hours to obtain the methanol solution of the α-epoxy-ω-amine hydrochloride intermediate;
[0039] S2: Add 3.04 grams (0.030 moles) of triethylamine to the above solution, turn on the stirring device, raise the temperature to reflux temperature, and continue the reaction for 32 hours to obtain a hyperbranched polythioether polyamine hydrochloride solution;
[0040] S3: Add 0.81 g (0.015 mol) of sodium methoxide to the hyperbranched polythioether polyamine hydrochlorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com