Vesicles consisting of amphiphilic polymer and application of vesicles
A polymer and amphiphilic technology, which is applied to vesicles composed of amphiphilic polymers and their application fields, can solve the problems of inability to protect proteins, low protein encapsulation efficiency, and limited biomedical applications of polymer vesicles.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
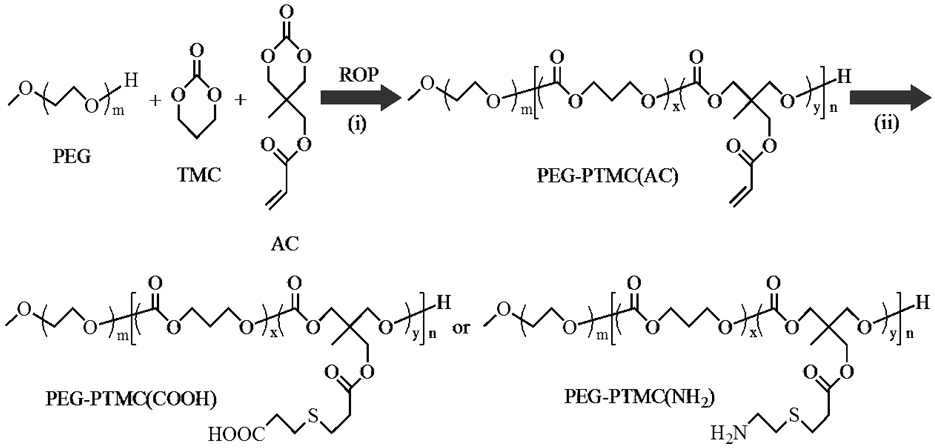
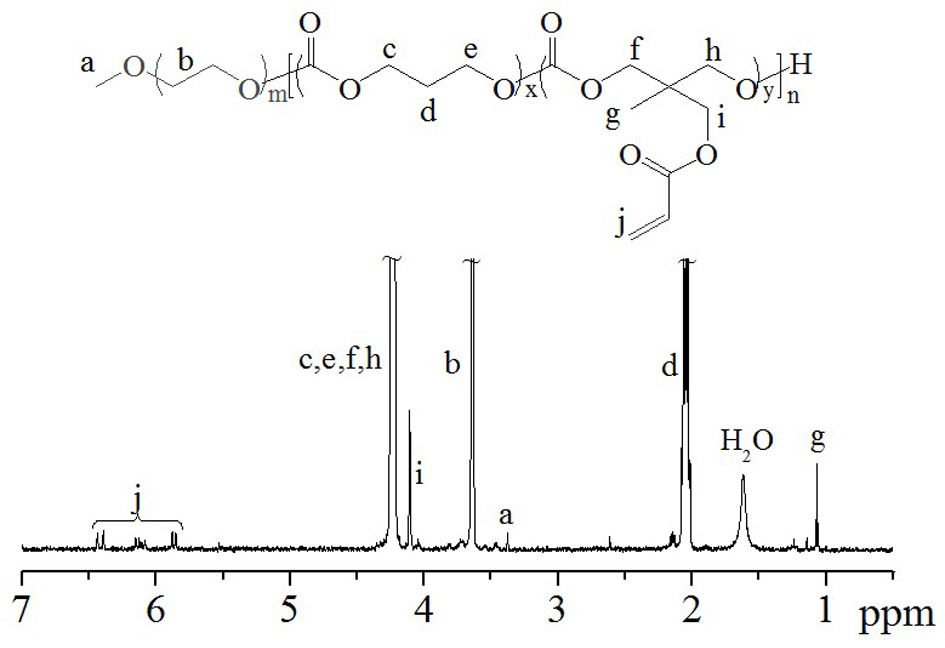
[0053] like figure 1 The shown ring-opening reaction obtains the block polymer PEG-PTMC (AC): under nitrogen protection, bis(bistrimethylsilyl)amine zinc catalyst (0.02g, 0.055mM), macroinitiator PEG (0.51g , 0.12mmol), TMC (1.80g, 0.018mol), AC (0.20g, 0.001mol) and 25mL of methylene chloride were added to a 50mL round bottom flask, operated in a glove box, and stirred at room temperature for 24 hours, After the reaction was completed, it was precipitated and filtered in ether, and then vacuum-dried for 48 hours to obtain a block polymer with a yield of 95%. From its proton nuclear magnetic test results ( figure 2 ) can be clearly seen in PEG ( CH 3 -O-: δ 3.38 and - CH 2 - CH2 -O-: δ 3.63), PTMC (-OCO- CH 2 -CH 2 - CH 2 -OCO-: δ 4.16 and -OCO-CH 2 - CH 2 -CH 2 -OCO-: δ 2.06) and PAC (-OCO- CH 2 -C- CH 2 -OCO-: δ 4.16, -C- CH 2 -OCO-: δ 4.13, CH 3 -C-: δ 1.07 and -OCO- CH 2 =CH 2 : δ 6.47, 6.15 and 5.85) characteristic peaks, indicating...
Embodiment 2
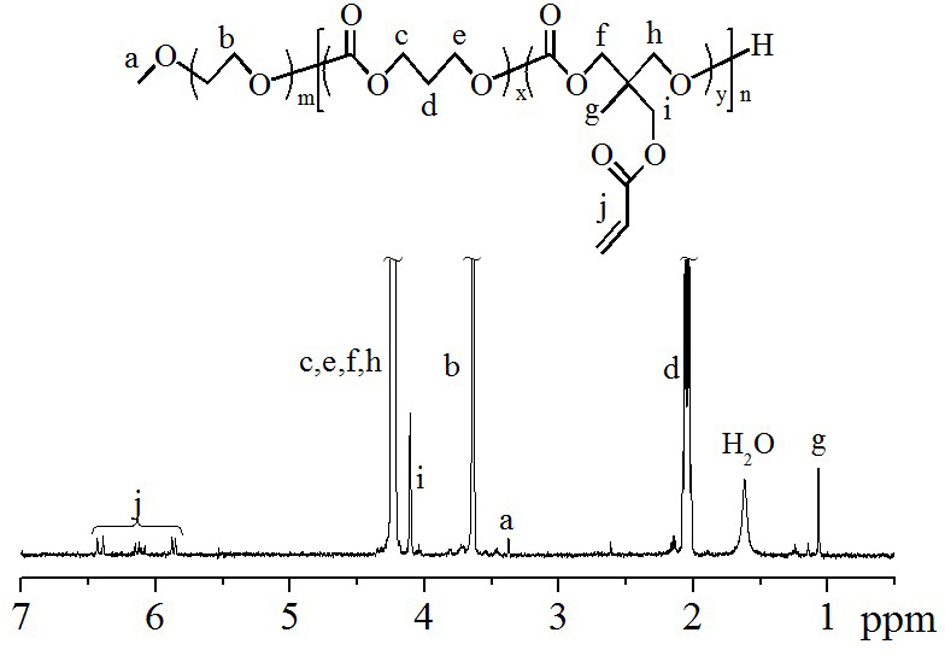
[0055] like figure 1 The shown Michael addition reaction obtained block polymer PEG-PTMC (COOH): under nitrogen protection, 3-mercaptopropionic acid (85mg, 0.800mM), pyridine (62mg, 0.800mM), polymer PEG-PTMC (AC ) (200mg, 0.008mM) and 2mL of N,N-dimethylformamide were added to a 10mL Schlenk vacuum-sealed reactor, filled with nitrogen for 0.5 hours, and then stirred at room temperature for 2 to 3 days. The mixed solution of cold ether and ethanol (volume ratio 4 / 1) was precipitated and filtered, and then vacuum-dried for 48 hours to obtain a block polymer with a yield of 67%. From its proton nuclear magnetic test results ( image 3 ) can be clearly seen in PEG ( CH 3 -O-: δ 3.38 and - CH 2 - CH 2 -O-: δ 3.63), PTMC (-OCO- CH 2 -CH 2 - CH 2 -OCO-: δ 4.16 and -OCO-CH 2 - CH 2 -CH 2 -OCO-: δ 2.06), PAC (-OCO- CH 2 -C- CH 2 -OCO-: δ 4.16, -C- CH 2 -OCO-: δ 4.13 and CH 3 -C-: δ 1.07) and COOH (-S- CH 2 - CH 2 -COOH: d 2.68-2.97) characterist...
Embodiment 3
[0057] like figure 1 The shown Michael addition reaction gave the block polymer PEG-PTMC (COOH) (3 COOH): under nitrogen protection, 3-mercaptopropionic acid (8.5mg, 0.080mM), pyridine (6.2mg, 0.080mM), Polymer PEG-PTMC (AC) (200mg, 0.008mM) and 2mL of N,N-dimethylformamide were added to a 10mL Schlenk vacuum-sealed reactor, filled with nitrogen for 0.5 hours, and then stirred at room temperature for 2~ After 3 days, after the reaction, it was precipitated and filtered in a mixed solution of cold ether and ethanol (volume ratio 4 / 1), and then vacuum-dried for 48 hours to obtain a block polymer with a yield of 67%. From its proton nuclear magnetic test results, it can be clearly seen that PEG ( CH 3 -O-: δ 3.38 and - CH 2 - CH 2 -O-: δ 3.63), PTMC (-OCO- CH 2 -CH 2 - CH 2 -OCO-: δ 4.16 and -OCO-CH 2 - CH 2 -CH 2 -OCO-: δ 2.06) and PAC (-OCO- CH 2 -C- CH 2 -OCO-: δ 4.16, -C- CH 2 -OCO-: δ 4.13, CH 3 -C-: δ 1.07 and -OCO- CH 2 =CH 2 : δ 6.47...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com