Magnetic nanometer material, and preparation method and application thereof in enrichment analysis for glycosylation peptide fragment
A magnetic nanometer and magnetic nanoparticle technology, applied in the fields of biotechnology and nanomaterials, achieves the effects of high sensitivity, good selectivity and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
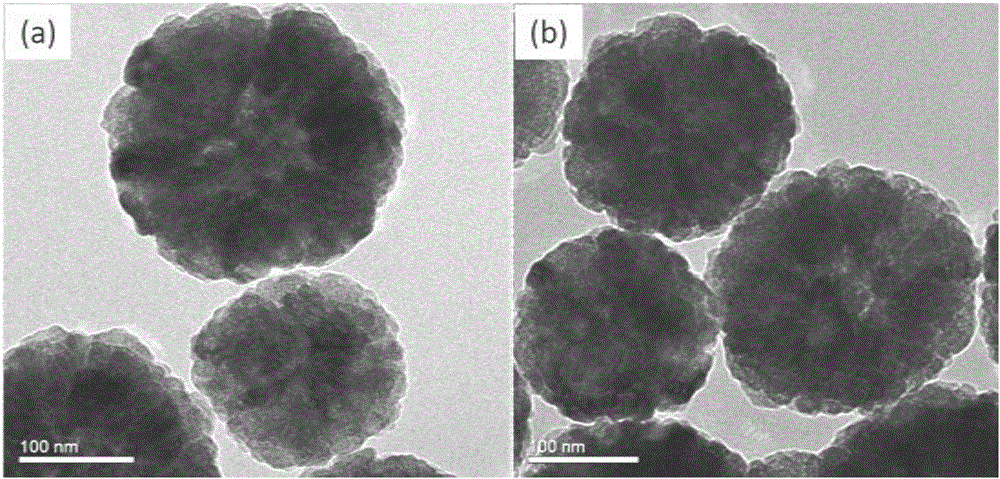
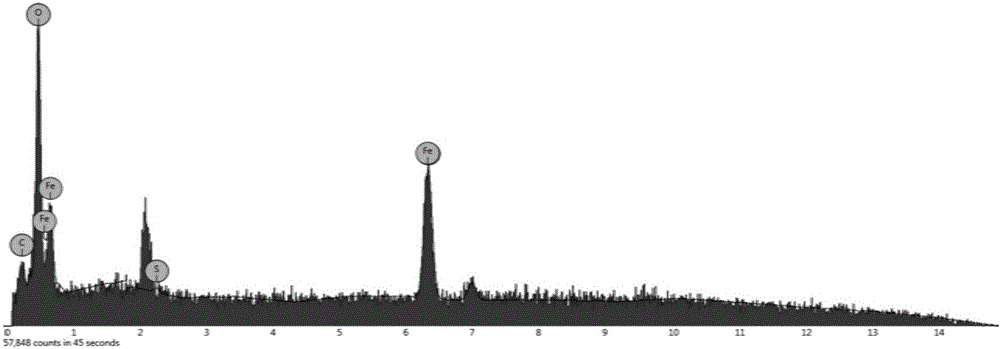
[0027] Example 1: Synthesis of a ferroferric oxide magnetic nanomaterial modified with cysteamine hydrochloride.
[0028] (1) Weigh 2.6g of ferric chloride hexahydrate and disperse it in 145mL of ethylene glycol, stir and mix evenly with magnetic force, add 7.3g of anhydrous sodium acetate to the mixture, continue to stir and mix evenly, and then disperse with ultrasound for 0.5 hours , transfer the mixed solution to a polytetrafluoroethylene-lined stainless steel reaction kettle, react at 200°C for 16 hours, then fully wash with deionized water and absolute ethanol, and dry under vacuum at 50°C to obtain ferric oxide magnetic nanoparticles.
[0029] (2) Weigh 22 mg ferroferric oxide magnetic nanoparticles obtained in step (1), and disperse them into 30 mL of 0.01M phosphate buffer, then add 88 mg of cysteamine hydrochloride, ultrasonically disperse for 5 minutes, and place in a water bath at 60°C After mechanical stirring for 3 hours, the product was magnetically separated f...
Embodiment 2
[0034] Example 2: The ferric iron tetroxide magnetic nanomaterial modified with cysteamine hydrochloride obtained in Example 1 was applied to the enrichment and MALDI-TOF MS detection of low-concentration horseradish peroxidase HRP hydrolyzate.
[0035] (1) Prepare standard proteolysis solution: Accurately weigh 1 mg of HRP standard protein, prepare a standard protein solution with a concentration of 10 mg / mL with 25 mM ammonium bicarbonate solution, boil for 5 minutes; then dilute with 25 mM ammonium bicarbonate solution to make HRP The final concentration is 1mg / mL, according to the mass ratio of 1:40 trypsin and standard protein, add trypsin (trypsin), and incubate at 37°C for 16 hours to obtain 1mg / mL HRP trypsin hydrolyzate.
[0036] (2) Enrichment of samples: Prepare a sample solution with a volume fraction of 85% acetonitrile / 14.9% water / 0.1% trifluoroacetic acid, and use the sample solution to prepare 10 mg / mL cysteamine hydrochloride-modified trioxide Dispersions of f...
Embodiment 3
[0042] Example 3: The ferric iron tetroxide magnetic nanomaterial modified by cysteamine hydrochloride obtained in Example 1 was enriched and eluted for many times, and then applied to the enrichment of low-concentration horseradish peroxidase HRP enzymatic hydrolyzate Set with MALDI-TOF MS detection.
[0043] (1) Prepare standard proteolysis solution: Accurately weigh 1 mg of HRP standard protein, prepare a standard protein solution with a concentration of 10 mg / mL with 25 mM ammonium bicarbonate solution, boil for 5 minutes; then dilute with 25 mM ammonium bicarbonate solution to make HRP The final concentration is 1mg / mL, according to the mass ratio of 1:40 trypsin and standard protein, add trypsin (trypsin), and incubate at 37°C for 16 hours to obtain 1mg / mL HRP trypsin hydrolyzate.
[0044] (2) Enrichment of samples: Prepare a sample solution with a volume fraction of 85% acetonitrile / 14.9% water / 0.1% trifluoroacetic acid, and use the sample solution to prepare 10 mg / mL c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com