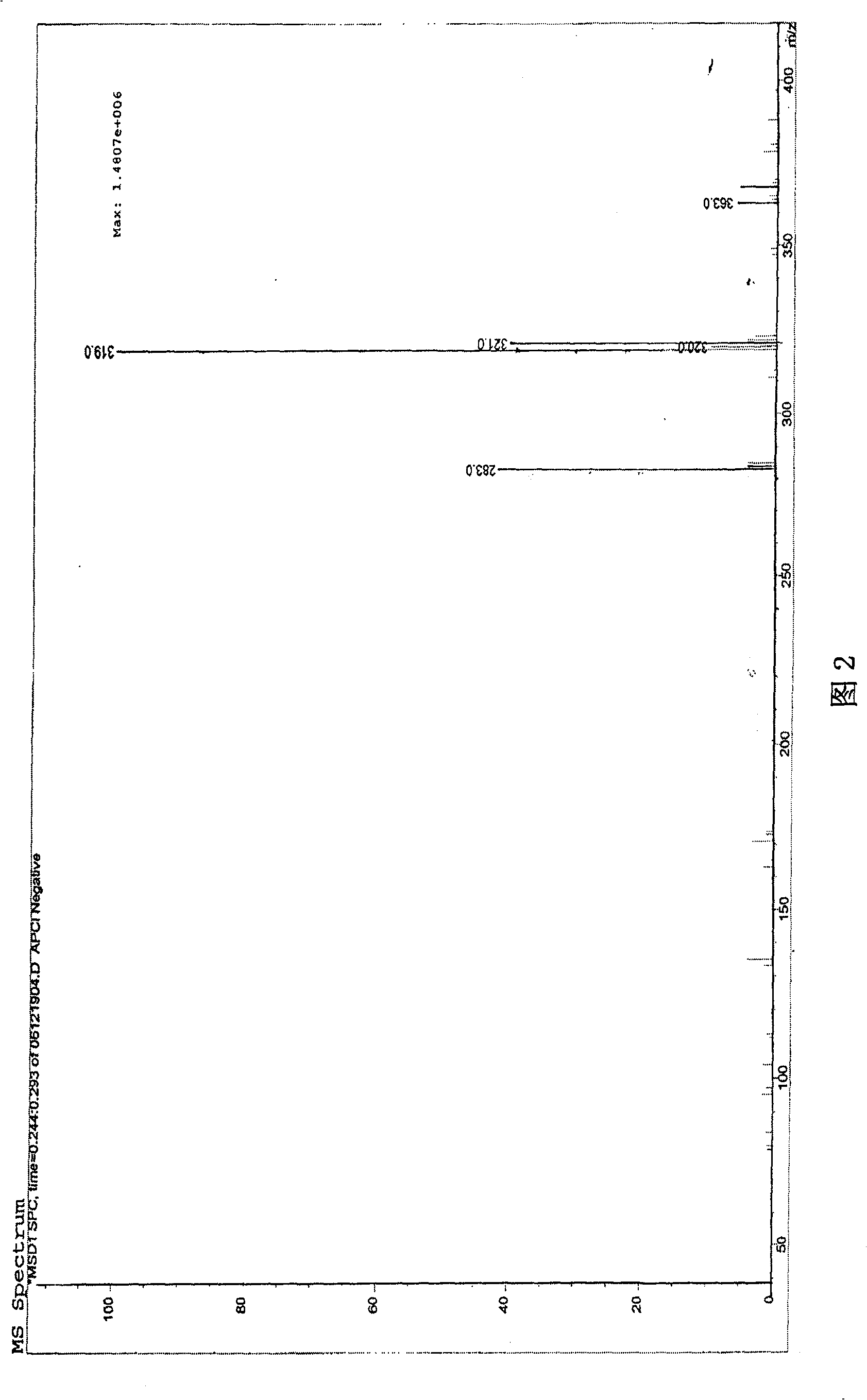
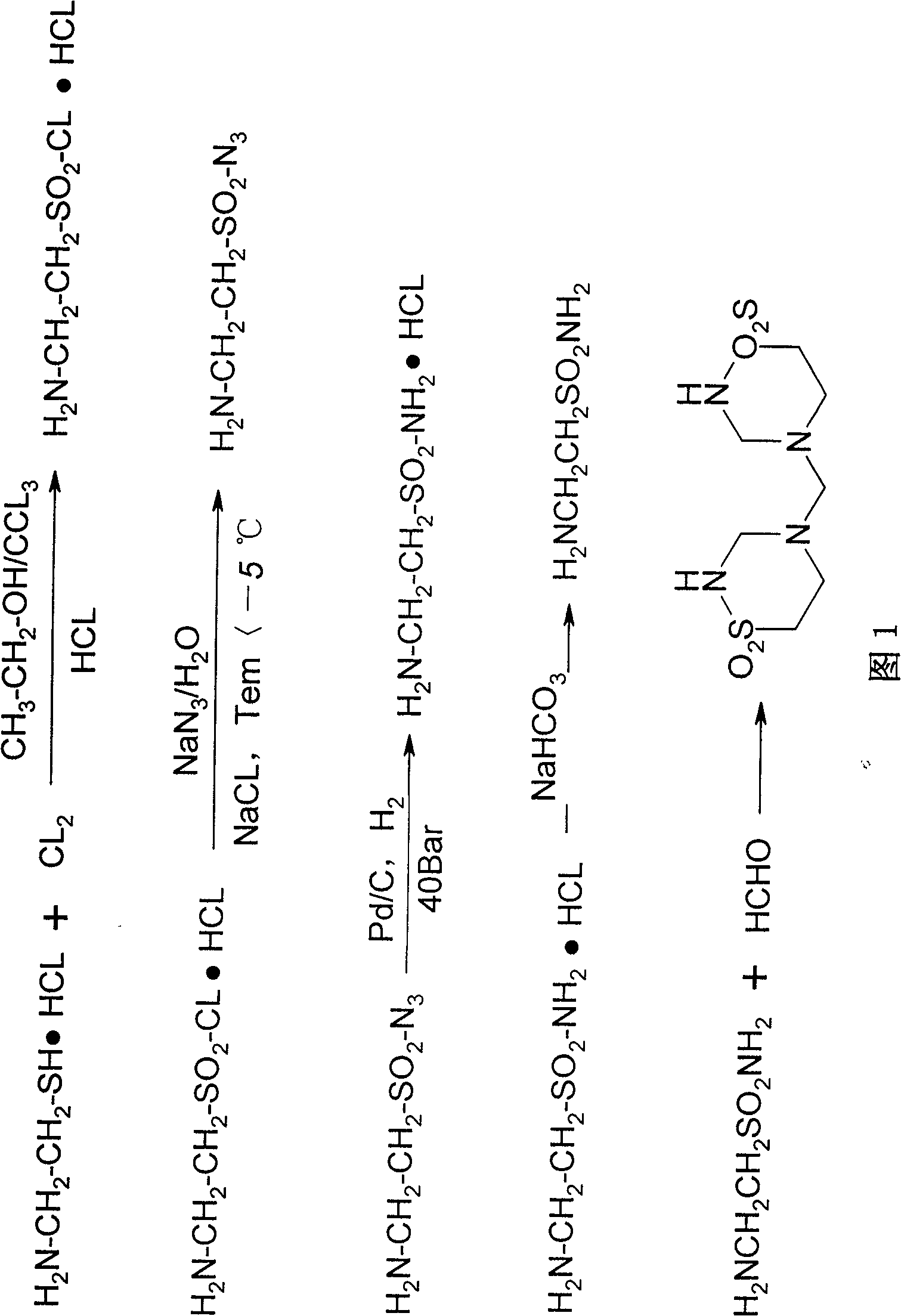Synthetic method for taurolidine and pharmaceutical preparations
A technology of taurolidine and a synthesis method, which is applied in the field of medicinal chemistry, can solve the problems affecting the quality of medicines, many chemical raw materials, and many reaction steps, and achieves the effects of exact curative effect, high purity and stable preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Taurolidine synthesis operation steps:
[0036] 1. Preparation of taurine chloride hydrochloride
[0037]
[0038] Add 25g of cysteamine hydrochloride, 200ml of dichloromethane and 32ml of absolute ethanol into a 300ml four-necked bottle equipped with a ventilation tube, a gas outlet tube, a thermometer and a mechanical stirrer. Under mechanical stirring in an ice-water bath (below 10°C), pass Add dry moderate chlorine gas, the reaction starts immediately and exothermic, a white viscous solid is formed, keep the temperature below 50°C and stir for 5 hours. The whole process uses lye to absorb HCl gas and ethyl chloride gas in the reaction process. After the reaction, the chlorine gas flow was stopped to obtain a yellow precipitate, which was filtered by suction, washed four times with dichloromethane, and dried in vacuo to obtain 50 g of a white solid with a melting point of 152-154°C.
[0039] 2. Preparation of tauryl azide hydrochloride
[0040]
[0041] Cool...
Embodiment 2
[0050] Refining of taurolidine:
[0051] Add 50-200ml of acetonitrile to 5-10g of the white taurolidine powder obtained above, heat to dissolve, filter to remove a small amount of insoluble matter, concentrate, and cool below 10°C to obtain 5-10g of white powder with a melting point of 172-174°C.
Embodiment 3
[0053] H NMR spectrum ( 1 H-NMR) data are as follows:
[0054] 1 HNMR (DMSO-D6, TMS7.26-7.28 (t, 2H, NH), 4.09-4.10 (d, 4H, N-CH 2 ), 3.53 (s, 2H, N-CH 2 -N), 3.28-3.29(t, 4H, N-CH 2 -CH 2 ), 2.96-2.97 (t, 4H, S-CH 2 -CH 2 ).
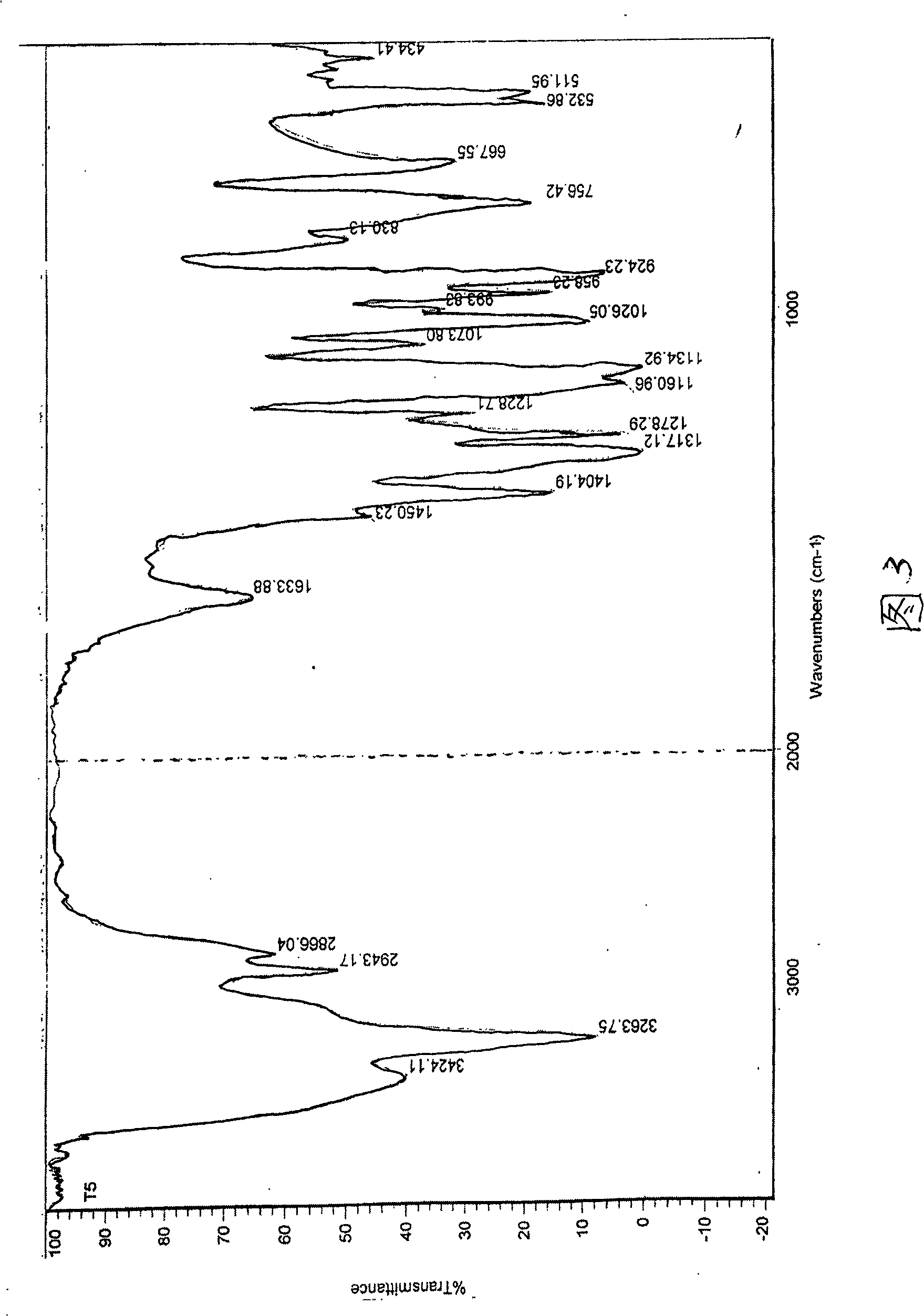
[0055] The infrared absorption spectrum data are as follows:
[0056] IR (KBr tablet cm -1): 3425, 3263, 1633, 1450, 1404, 1317, 1278, 1228, 1160, 1134, 1073, 1026, 993, 958, 924, 830, 757, 667, 532, 511. See Figure 3.
[0057] Elemental analysis analysis value:
[0058] C, 29.04%, N, 18.55%, H, 5.85%; Calculated: C, 29.57%, N, 19.71%, H, 5.67%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com