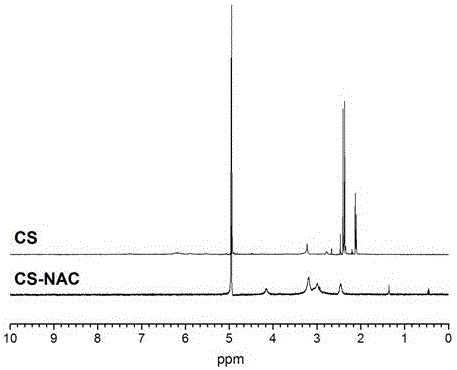
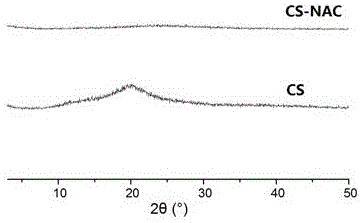
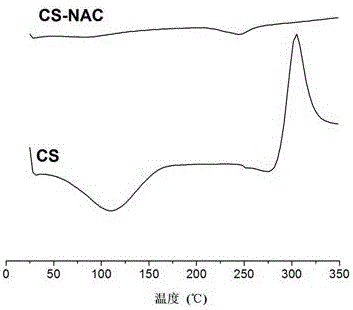
Novel bioadhesive thiolated chitosan synthesis method
A technology of thiolated chitosan and a synthesis method, which is applied in the synthesis field of novel bioadhesive thiolated chitosan, can solve the problems of reducing the catalytic activity of P-gp, etc., so as to ensure the exertion of efficiency, avoid hydrolysis and reaction controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Dissolve 2 g of N-acetyl-L-cysteine (NAC), EDC·HCl, and HOBT (molar ratio 4:1:1) in 10 mL N,N-dimethylformamide ( DMF), stirring continuously at room temperature for 3-6 hours to activate NAC.
[0028] (2) Dissolve CS in 20% hydrochloric acid solution to prepare a polymer solution with a concentration of 1.25% (w / v).
[0029] (3) Add the activated NAC to the CS solution (the molar ratio of NAC to CS is 4:1), adjust the pH to 5 with NaOH, and react in the dark for 3-6 hours at room temperature.
[0030] (4) To purify the product, remove unreacted NAC, dialyze the reaction solution once with hydrochloric acid solution containing 5mmol / L, dialyze twice with hydrochloric acid (5mmol / L) containing 1% NaCl, and then dialyze the reaction solution with hydrochloric acid solution containing 5mmol / L The hydrochloric acid solution was dialyzed twice, and the time of each dialysis was 12 hours, and the whole dialysis process was carried out at 4°C in the dark. The dialyzed ...
Embodiment 2
[0033] (1) Dissolve 2 g of cysteine, EDC·HCl, and HOBT (molar ratio 10:9:1) in 10 mL N,N-dimethylformamide (DMF), and stir continuously at room temperature for 3 -6 hours to activate cysteine.
[0034] (2) Dissolve CS in 10% hydrochloric acid solution to prepare a polymer solution with a concentration of 0.8% (w / v).
[0035] (3) Add the activated cysteine to the CS solution (the molar ratio of cysteine to CS is 20:1), adjust the pH to 4 with NaOH, and react in the dark for 3-6 hours at room temperature.
[0036] (4) To purify the product, remove unreacted NAC, dialyze the reaction solution once with hydrochloric acid solution containing 1mmol / L, dialyze twice with hydrochloric acid (1mmol / L) containing 0.5% NaCl, and then dialyze the reaction solution with hydrochloric acid solution containing 1mmol / L The hydrochloric acid solution was dialyzed twice, and the time of each dialysis was 12 hours, and the whole dialysis process was carried out at 4°C in the dark. The dialyz...
Embodiment 3
[0039] (1) Dissolve 2 g of thioglycolic acid (TGA), EDC·HCl, HOBT (molar ratio 10:0.5:8) in 10 mL N,N-dimethylformamide (DMF), and stir continuously at room temperature 3-6 hours to activate TGA.
[0040] (2) Dissolving CS in 20% hydrochloric acid solution to prepare a polymer solution with a concentration of 1% (w / v).
[0041](3) Add the activated TGA to the CS solution (the molar ratio of TGA to CS is 10:1), adjust the pH to 5 with NaOH, and react in the dark for 3-6 hours at room temperature.
[0042] (4) To purify the product, remove unreacted TGA, dialyze the reaction solution once with hydrochloric acid solution containing 3mmol / L, dialyze twice with hydrochloric acid (3mmol / L) containing 1% NaCl, and then dialyze the reaction solution with hydrochloric acid solution containing 3mmol / L The hydrochloric acid solution was dialyzed twice, and the time of each dialysis was 12 hours, and the whole dialysis process was carried out at 4°C in the dark. The dialyzed solution is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com