Chitosan/polylysine dendritic macromolecular core-shell nanoparticles and preparation method thereof
A technology of dendritic macromolecule and polylysine, which is applied in the direction of nanotechnology, medical preparations of non-active ingredients, pharmaceutical formulas, etc., can solve the problems of low bioavailability and high cytotoxicity of drugs, and improve bioavailability The effect of high degree, simple preparation method and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
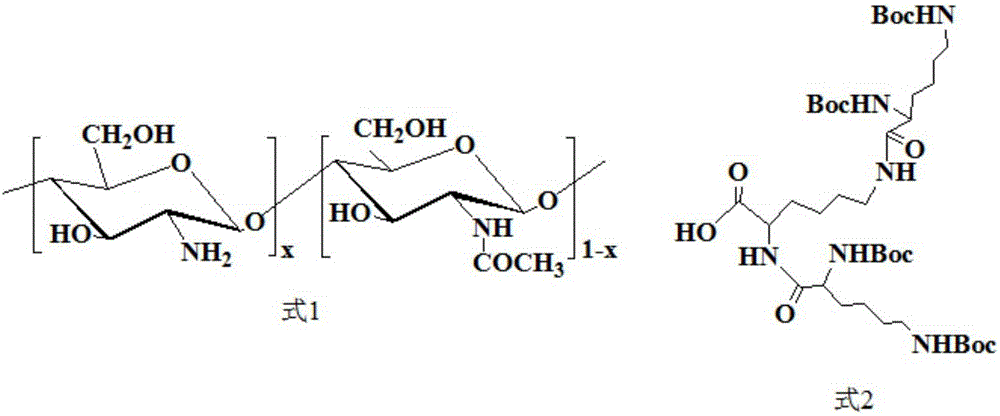
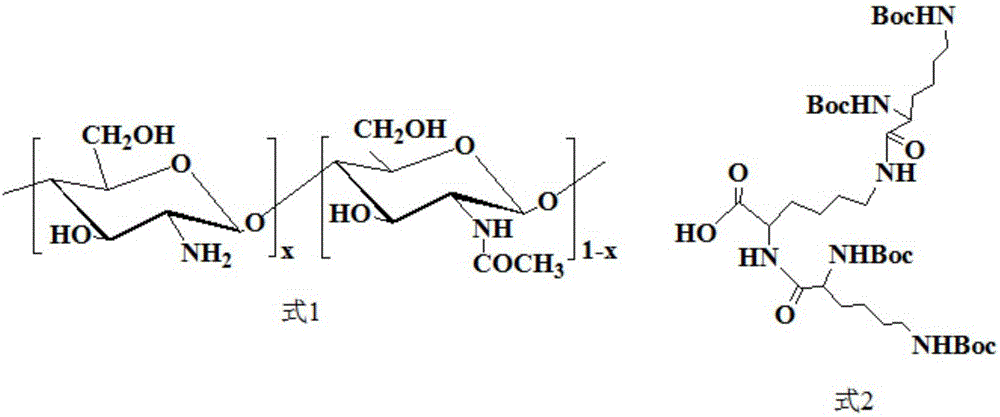
[0029] At 25°C, under nitrogen protection and stirring at 600 rpm, 5 g of methyl ester-protected lysine, 18.5 g of Boc-protected lysine, 16.4 g of EDC and 6.3 g of HOBt were dissolved in 20 ml of dichloromethane. While continuing to stir, slowly add 7ml of N-ethyldiisopropylamine. After that, the reaction was carried out for 48 hours at 25° C. under nitrogen protection and stirring at 600 rpm. After the reaction was finished, the solvent dichloromethane was removed by rotary evaporation. The residue was dissolved in about 400ml of chloroform, washed once with about 350ml of saturated NaCl solution, washed once with about 350ml of saturated NaHCO 3 Solution washed 3 times, 350ml 1mol / l dilute hydrochloric acid solution washed 1 time and 350ml saturated NaCl solution washed 1 time. The obtained oil phase was treated with anhydrous MgSO 4 Dry overnight, filter, and rotary evaporate, and then use silica gel column chromatography (eluent is a mixed solvent of dichloromethane / eth...
Embodiment 2
[0033] The preparation process of Boc-protected 2nd generation polylysine dendrimer (G2-Boc) is the same as that in Example 1.
[0034] Under nitrogen protection at 25°C and stirring at 600 rpm, 5 g of the obtained Boc-protected 2nd-generation polylysine dendrimer (G2-Boc) was dissolved in 10 ml of redistilled dichloromethane. After that, 18.8 ml of trifluoroacetic acid was slowly added in an ice-water bath at 2° C. while stirring was continued. After that, the reaction was stirred for 5 h at 25° C. under the protection of nitrogen. After the reaction, dichloromethane and trifluoroacetic acid were removed by rotary evaporation. The residue was precipitated with about 80ml of anhydrous ether and stirred overnight, and the anhydrous ether was removed by rotary evaporation to obtain a 2nd generation polylysine dendrimer (G2-NH 2 ).
[0035] 25 DEG C, under nitrogen protection, under the condition of 600 rpm stirring, the 2.55g de-Boc-protected 2nd generation polylysine dendrim...
Embodiment 3
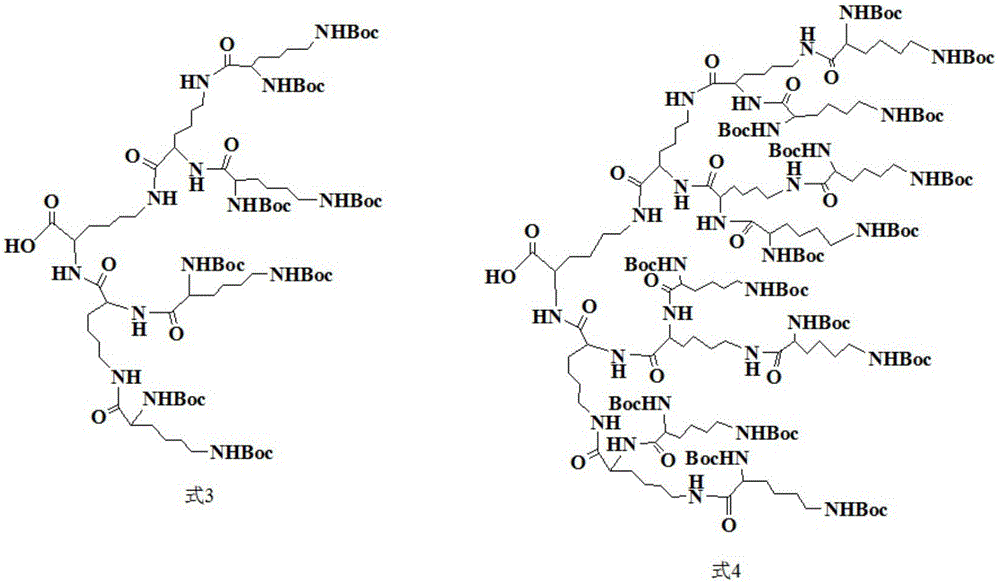
[0039] The preparation process of Boc-protected third-generation polylysine dendrimer (G3-Boc) is the same as that in Example 2.
[0040] 5 g of the obtained Boc-protected third-generation polylysine dendrimer (G3-Boc) was dissolved in 10 ml of N,N-dimethylformamide at 25° C. under nitrogen protection and stirring at 600 rpm. Afterwards, in an ice-water bath at 2°C, 9 ml of trifluoroacetic acid was slowly added under continuous stirring. Then, at 25°C, under the protection of nitrogen, the reaction was stirred for 5 h. After the reaction, N,N-dimethylformamide and trifluoroacetic acid were removed by rotary evaporation. The residue was precipitated with about 50ml of anhydrous ether and stirred overnight, and the anhydrous ether was removed by rotary evaporation to obtain a third-generation polylysine dendrimer (G3-NH 2 ).
[0041] 25 DEG C, under nitrogen protection, under the condition of 600 rpm stirring, the 2.68g de-Boc-protected 3rd generation polylysine dendrimer (G3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of deacetylation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com