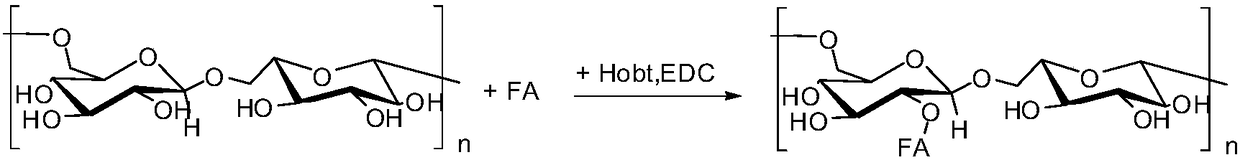Preparation method of polysaccharide grafted folic acid copolymer and nanoparticle thereof
A technology of copolymer and branch folic acid, which is applied in the directions of pharmaceutical combinations, pharmaceutical formulations, medical preparations of inactive ingredients, etc., can solve the problems of organic solvent residue, long preparation period, toxicity, etc., and achieves short operation time and good biological safety. performance, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Dextran grafted folic acid copolymer has the following general formula:
[0061]
[0062] n is 640 and the molecular weight of dextran is 40k Da.
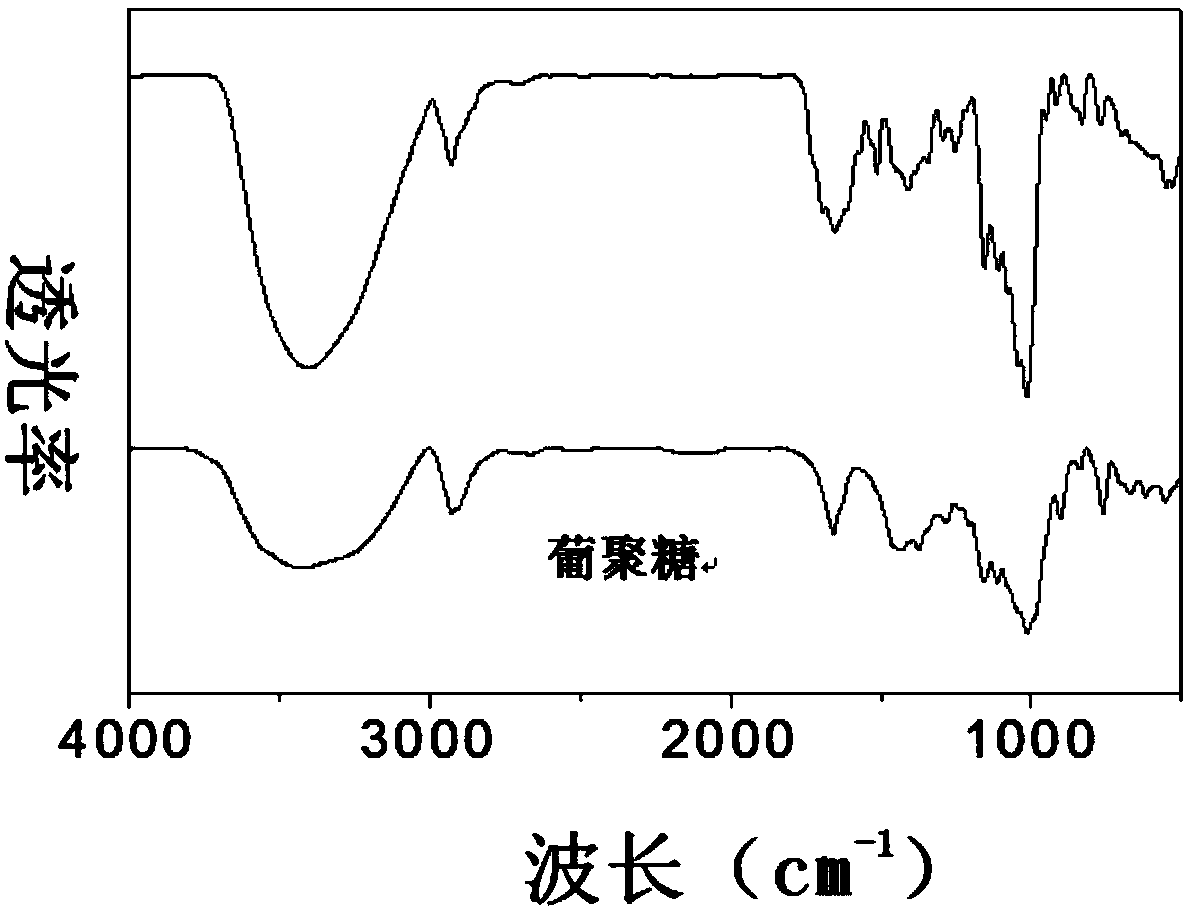
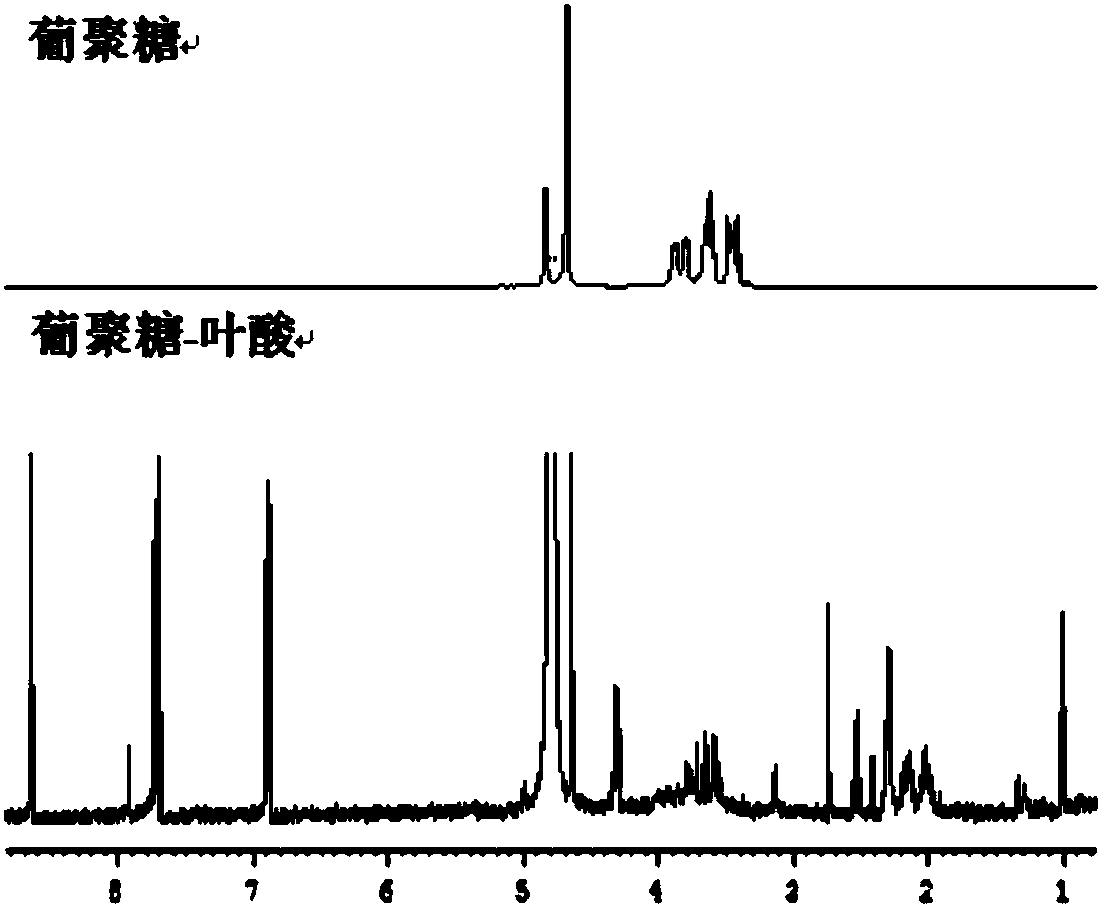
[0063] Such as figure 1 The preparation method of shown above-mentioned dextran-grafted folic acid copolymer comprises the following steps:
[0064] 1) Dissolving folic acid and activating its carboxyl group: dissolve 0.5g folic acid with 5ml of anhydrous dimethyl sulfoxide, then add 1-hydroxybenzotriazole and N-N'-dicyclohexylcarbodiimide, at a temperature of 60 Carboxyl activation reaction at ℃ for 30 minutes, stirring at room temperature for 2-4 hours to obtain a carboxyl-terminated folic acid solution; wherein, folic acid, 1-hydroxybenzotriazole and N-N'-dicyclohexylcarbodiimide The feeding molar ratio is 1:1:1;
[0065] 2) Dissolving dextran: under the protection of helium, fully dissolve 0.5 g of dextran with a molecular weight of 40 kDa in 5 ml of anhydrous dimethyl sulfoxide at a temperature of 50 ° C to obtai...
Embodiment 2
[0081] Oligochitosan grafted folic acid copolymer has the following general formula:
[0082]
[0083] R is chitosan oligosaccharide, its molecular weight is 5kDa.
[0084] Such as Figure 5 The preparation method of shown above-mentioned chitosan oligosaccharide grafted folic acid copolymer comprises the following steps:
[0085] 1) Dissolving folic acid and activating its carboxyl group: dissolve 0.5g folic acid with 5ml of anhydrous dimethyl sulfoxide, then add 1-hydroxybenzotriazole and N-N'-dicyclohexylcarbodiimide, at a temperature of 60 Carboxyl activation reaction at ℃ for 30 minutes, stirring at room temperature for 2-4 hours to obtain a carboxyl-terminated folic acid solution; wherein, folic acid, 1-hydroxybenzotriazole and N-N'-dicyclohexylcarbodiimide The feeding molar ratio is 1:1:1;
[0086] 2) Dissolving polysaccharides: Under the condition of helium protection, fully dissolve 0.5 g of chitosan oligosaccharides with a molecular weight of 5 kDa in anhydrous...
Embodiment 3
[0102] Pullulan grafted folic acid copolymer has the following general formula:
[0103]
[0104] Wherein, R is pullulan; its molecular weight is 300kDa.
[0105] Such as Figure 9 The preparation method of shown above-mentioned dextran-grafted folic acid copolymer comprises the following steps:
[0106] 1) Dissolving folic acid and activating its carboxyl group: dissolve 0.5g folic acid with 5ml of anhydrous dimethyl sulfoxide, then add 1-hydroxybenzotriazole and N-N'-dicyclohexylcarbodiimide, at a temperature of 60 Carboxyl activation reaction at ℃ for 30 minutes, stirring at room temperature for 2-4 hours to obtain a carboxyl-terminated folic acid solution; wherein, folic acid, 1-hydroxybenzotriazole and N-N'-dicyclohexylcarbodiimide The feeding molar ratio is 1:1:1;
[0107] 2) Dissolving polysaccharides: Under the protection of helium, fully dissolve 0.5 g of pullulan with a molecular weight of 300 kDa in anhydrous dimethyl sulfoxide at a temperature of 50 ° C to ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com