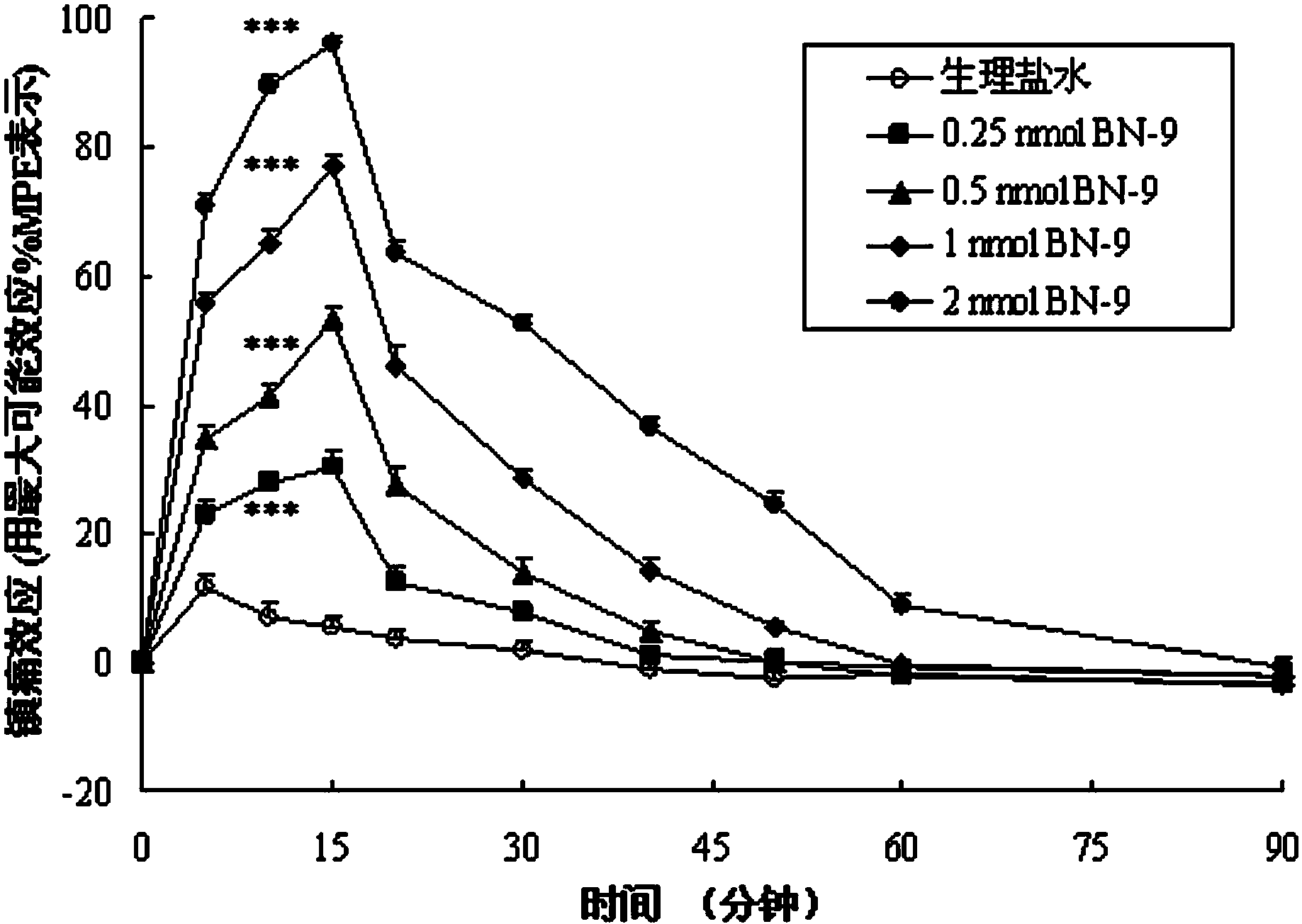
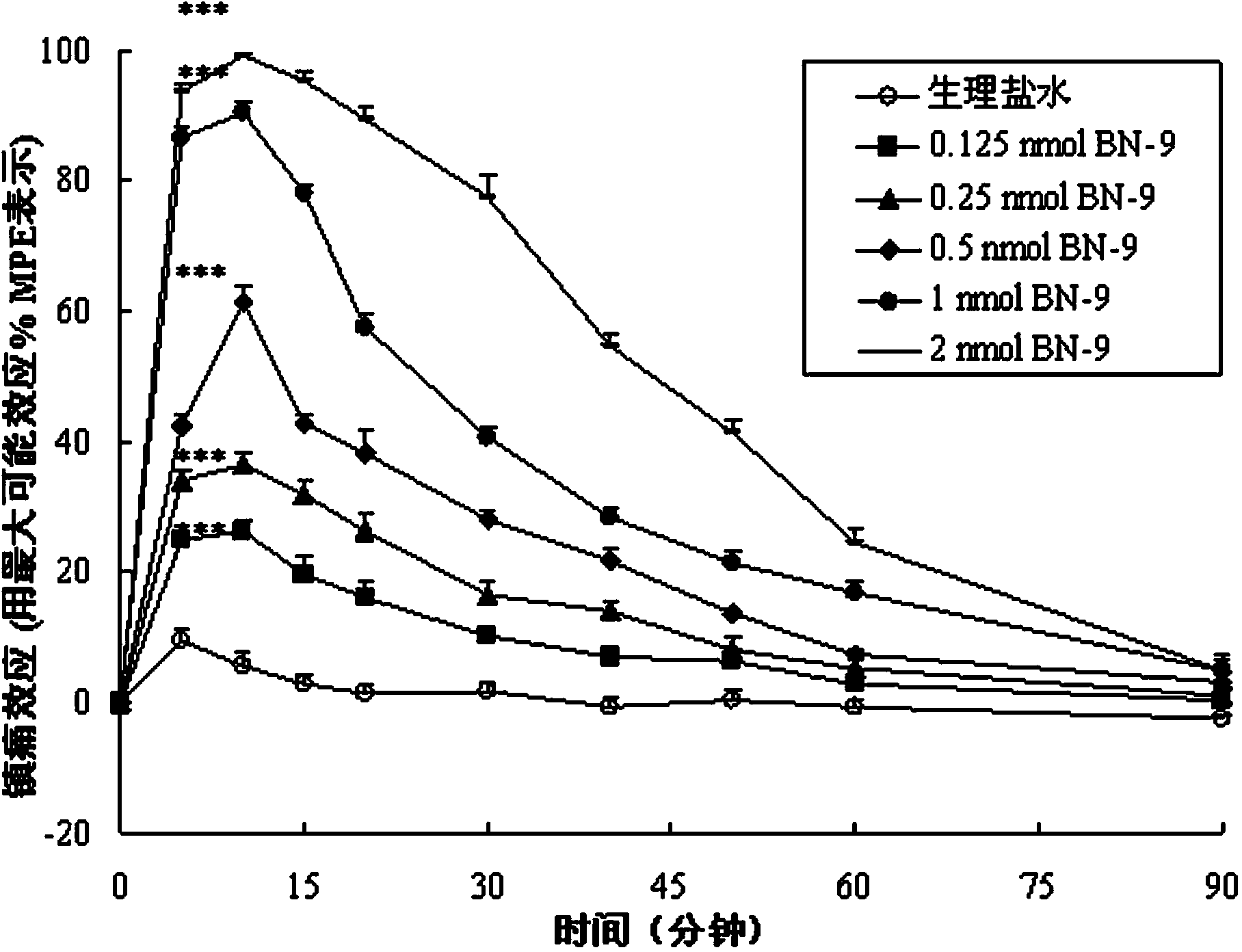
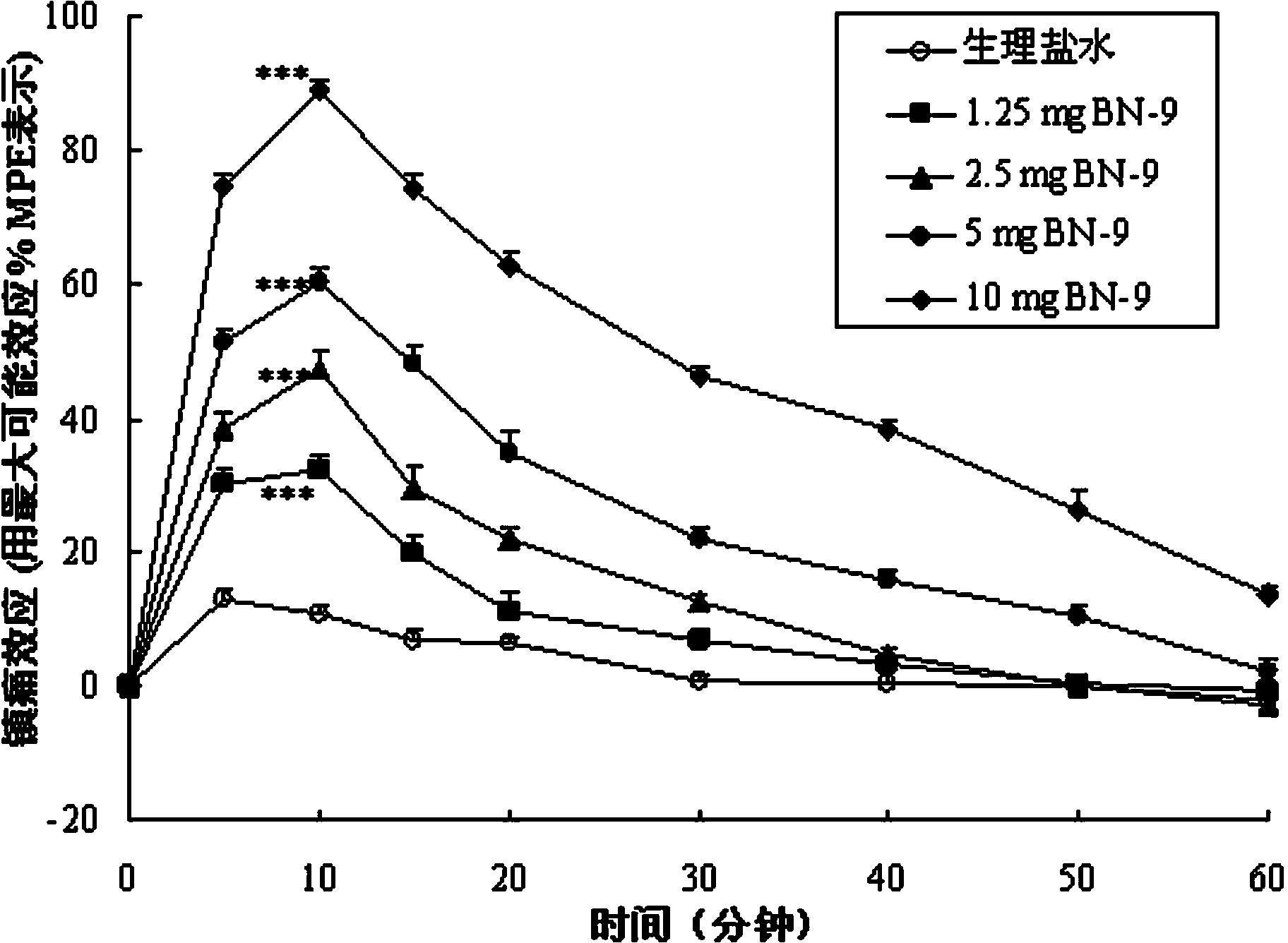
Chimeric peptide based on opioid peptide Biphalin and neuropeptide FF, synthesis and application thereof
A technology of chimeric peptides and opioid peptides, applied in the field of biochemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0082] The chimeric peptide synthesis method of the present invention will be further described through specific examples below.
[0083] I material
[0084] Instrument: High performance liquid chromatography (HPLC) is Delta 600 from Waters; analytical column: DELTA PAK 5μC18 300~3.9×150mm; preparative column: DELTA PAK 15μC18 300~7.8×300mm. The mass spectrometer was PE Biosystems, Mariner System 5074. Manual solid-phase peptide synthesizer, designed by our laboratory and made by a glass worker (for the design principle of the synthesizer, please refer to page 14 of "Fmoc solid phase peptide synthesis" edited by Chen WC and White PD Figure 4 , and some improvements were made on the basis of it, that is, the method of blowing nitrogen gas was replaced by mechanical stirring, so as to achieve the purpose of fully mixing the reaction solution). Reagent: The resin is Rink-Amide-MBHA-Resin (1% DVB, 200 ~ 400 mesh, substitution value S = 0.40 mmol / g resin), purchased from Tianjin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com