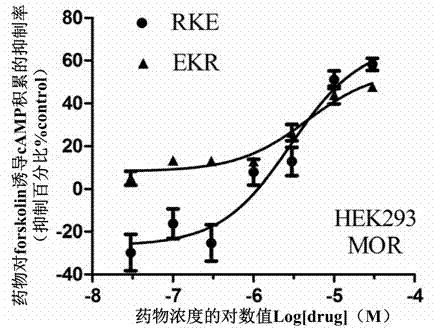
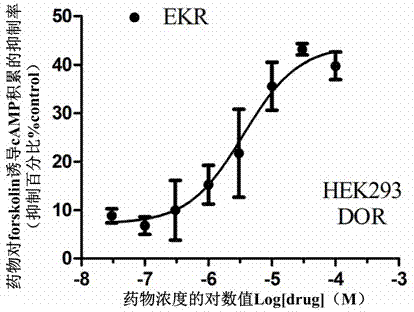
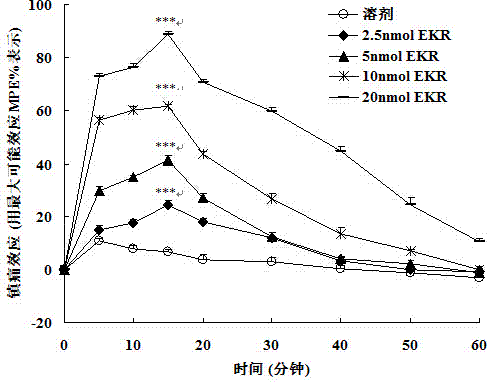
Split type hybrid peptides based on endomorphin 2 and NPFF (Neuropeptide FF) receptor antagonist RF9, as well as synthetic method and application thereof
A receptor antagonist, endomorphin technology, applied in the field of biochemistry, can solve the problems of limited clinical application, constipation, analgesic tolerance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] The synthesis of embodiment 1, EKR
[0092] (1) Resin pretreatment: Weigh 800 mg of Rink-Amide-MBHA resin with a substitution value of 0.43 mmol / g and add it to the synthesizer, then add DCM and stir for 30 min to fully swell the resin and dry the solvent under reduced pressure.
[0093](2) Removal of Fmoc group protection: Add a mixed solution of hexahydropyridine, DBU, and DMF (the volume ratio of the three is 1:1:98) to the resin that has been swollen and drained of solvent, stir for 5 minutes and then pump Dry, repeat 3 times and then drain. The DMF that was added at the end was stirred for 3 min and then sucked dry. This was repeated 4 times to obtain a resin sample from which the protection of the Fmoc group was removed.
[0094] (3) Indene detection: add to the test tube in the following order: resin sample, 0.1 ml reagent , 0.2ml reagent , 0.1 ml reagent , observe after boiling water bath for 3-10 min. Both the solution and the resin are blue, indicating...
Embodiment 2
[0103] Embodiment two, the synthesis of RKE
[0104] (1) Resin pretreatment: Add 500 mg of Rink-Amide-MBHA resin with a substitution value of 0.4 mmol / g into the synthesizer, and the treatment process is the same as in Example 1.
[0105] (2) Removal of Fmoc group protection: Same as Example 1.
[0106] (3) Indene detection: same as Example 1.
[0107] (4) Condensation of linker Lys: Same as Example 1.
[0108] (5) Main chain condensation: The peptide resin obtained in step (4) from which the Fmoc protective group has been removed is sequentially completed in steps (2) and (3) to complete Fmoc-Phe-OH, Fmoc-Arg(Pbf)-OH , Condensation of 1-adamantanic acid and deprotection of Fmoc group to obtain peptide resin sample 1-adamantanylation-Phe-Arg(Pbf)-Lys(Mtt)-Resin.
[0109] (6) Removal of the Lys side chain amino Mtt protecting group: After the resin obtained in step (5) is drained of solvent, add 1%-1.5% TFA / DCM solution, stir for 2 min and then drain, repeat 50 times Drain....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com