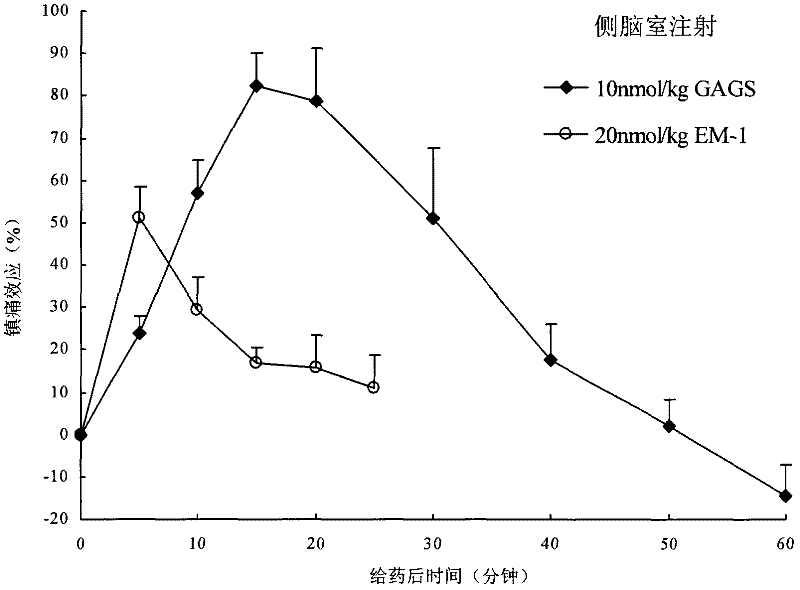
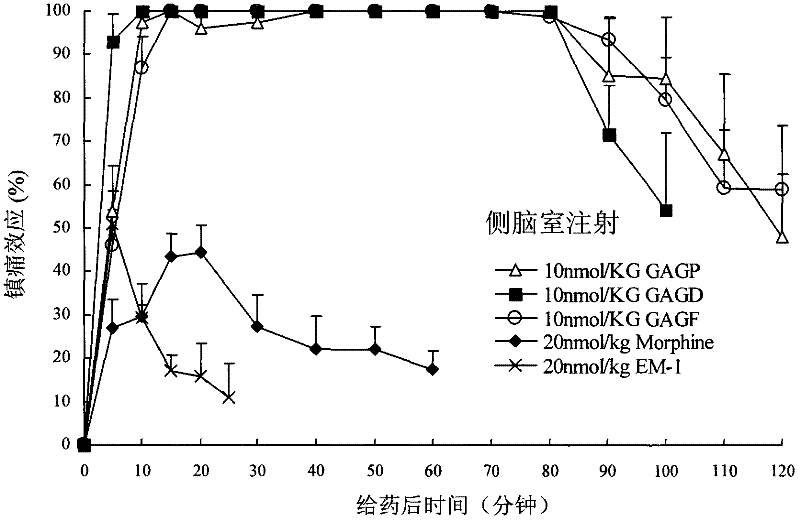
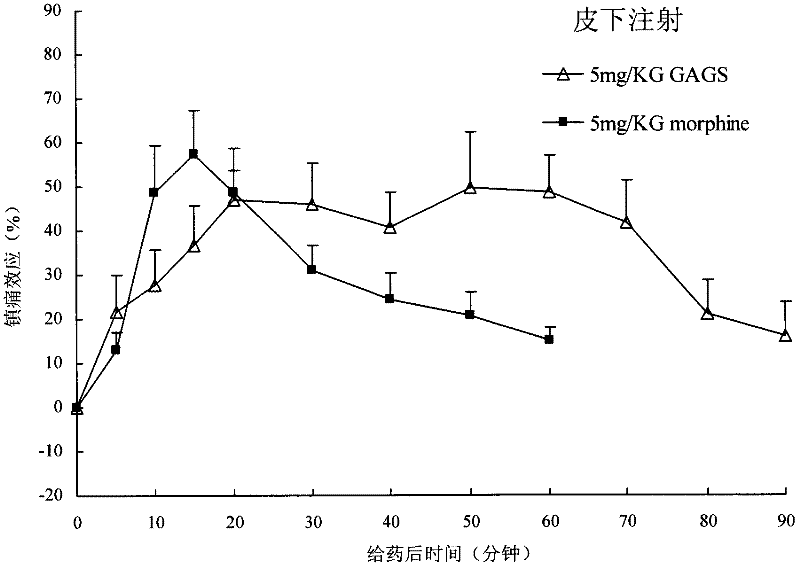
Endomorphin-1 analogs, synthesis thereof and application of endomorphin-1 analogs in preparation of analgesic medicines
A technology of endomorphin and analogs, applied in the field of biochemistry, can solve the problems of poor blood-brain barrier permeability and poor enzymatic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1: Synthesis of Analogue [GAGS]-EM-1
[0122] (1) Synthesis of monobenzyloxycarbonylamidinopyrazole
[0123] 0.73g (5mmol) compound amidinopyrazole hydrochloride joins in the mixed solution of equal volume dichloromethane (DCM) and dimethylformamide (DMF), adds triethylamine (10mmol), under ice bath slowly Add 845ul (6mmol) benzyloxycarbonyl chloride dropwise, react in ice bath for 30 minutes, and react at room temperature for 1.5 hours. After the reaction was complete, the DCM was concentrated under reduced pressure, a large amount of ethyl acetate was added to dissolve the residue, extracted three times with 5% citric acid aqueous solution and saturated NaCl aqueous solution, dried over anhydrous sodium sulfate, and concentrated. The crude product was crystallized from ethyl acetate / petroleum ether (1:4, v / v) to obtain 1.127 g of monobenzyloxycarbonylamidinopyrazole as white crystals. The yield was 92%. ESI-MS m / z=245[M+H] + .
[0124] (2) Synthesis of bi...
Embodiment 2
[0140] Example 2: Synthesis of Analogue [GAGP]-EM-1
[0141] (1) Synthesis of monobenzyloxycarbonylamidinopyrazole
[0142] With embodiment 1 step (1)
[0143] (2) Synthesis of bisbenzyloxycarbonylamidinopyrazole
[0144] With embodiment 1 step (2)
[0145] (3)Cbz 2 -Synthesis of Amidino-Tyr-D-Ala-OH
[0146] With embodiment 1 step (3)
[0147] (4)Cbz 2 -Amidino-Tyr-D-AIa-GIy-Phe(4-Cl)-NH 2 Synthesis
[0148] 0.562g (1mmol) of Cbz 2 - Amidino-Tyr-D-AIa-OH and 0.126g (1.1mmol) of HOSU were dissolved in THF and stirred at 0-5°C for 10 minutes; then 0.227g (1.1mmol) of DCC was added and Stir for 20 minutes, then stir the reaction at room temperature for 6 hours to give Cbz 2 -Activated ester solution of Amidino-Tyr-D-AIa-OH i.e. Cbz 2 - Amidino-Tyr-D-AIa-OSU solution.
[0149] 0.374g (1.1mmol) of Boc-Gly-Phe(4-Cl)-NH 2 Dissolve in 2ml of ethyl acetate, add 0.5ml of concentrated hydrochloric acid at 0-5°C, react for 30 minutes; then react at room temperature for 2 hou...
Embodiment 3
[0157] Example 3: Synthesis of Analogue [GAGF]-EM-1
[0158] (1) Synthesis of monobenzyloxycarbonylamidinopyrazole
[0159] With embodiment 1 step (1)
[0160] (2) Synthesis of bisbenzyloxycarbonylamidinopyrazole
[0161] With embodiment 1 step (2)
[0162] (3)Cbz 2 -Synthesis of Amidino-Tyr-D-Ala-OH
[0163] With embodiment 1 step (3)
[0164] (4)Cbz 2 -Amidino-Tyr-D-AIa-GIy-Phe(4-F)-NH 2 Synthesis
[0165] 0.562g (1mmol) of Cbz 2 - Amidino-Tyr-D-AIa-OH and 0.126g (1.1mmol) of HOSU were dissolved in THF and stirred at 0-5°C for 10 minutes; then 0.227g (1.1mmol) of DCC was added and Stir for 20 minutes, then stir the reaction at room temperature for 6 hours to give Cbz 2 -Activated ester solution of Amidino-Tyr-D-AIa-OH i.e. Cbz 2 - Amidino-Tyr-D-AIa-OSU solution.
[0166] 0.374g (1.1mmol) of Boc-Gly-Phe(4-F)-NH 2 Dissolve in 2ml ethyl acetate, add 0.5ml concentrated hydrochloric acid at 0-5°C, react for 30 minutes; then react at room temperature for 2 hours, conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com