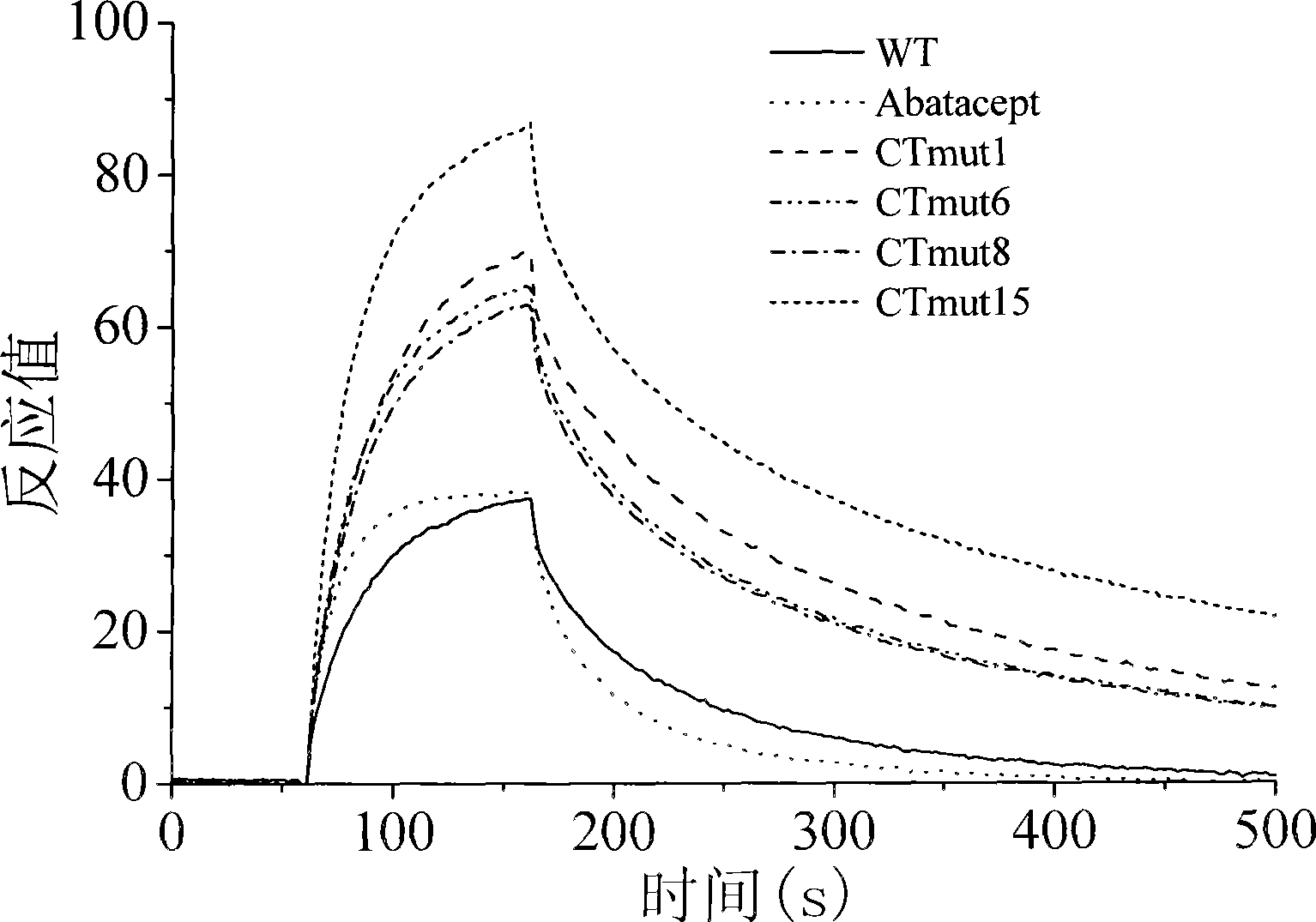
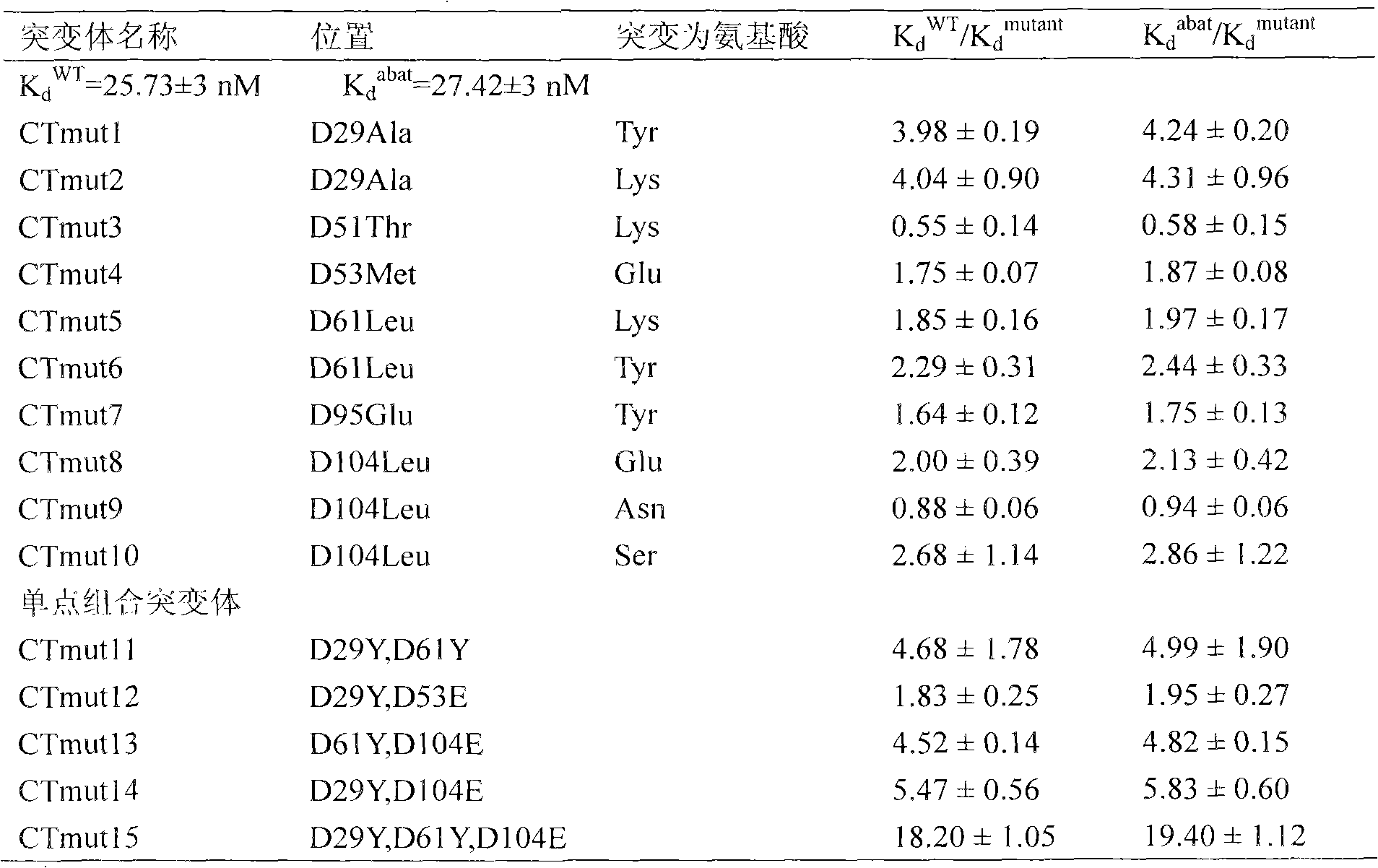
A high-affinity CTLA4-IG fusion protein mutant
A fusion protein, mutant technology, applied in the field of genetic engineering products, can solve the problem of long retention time and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
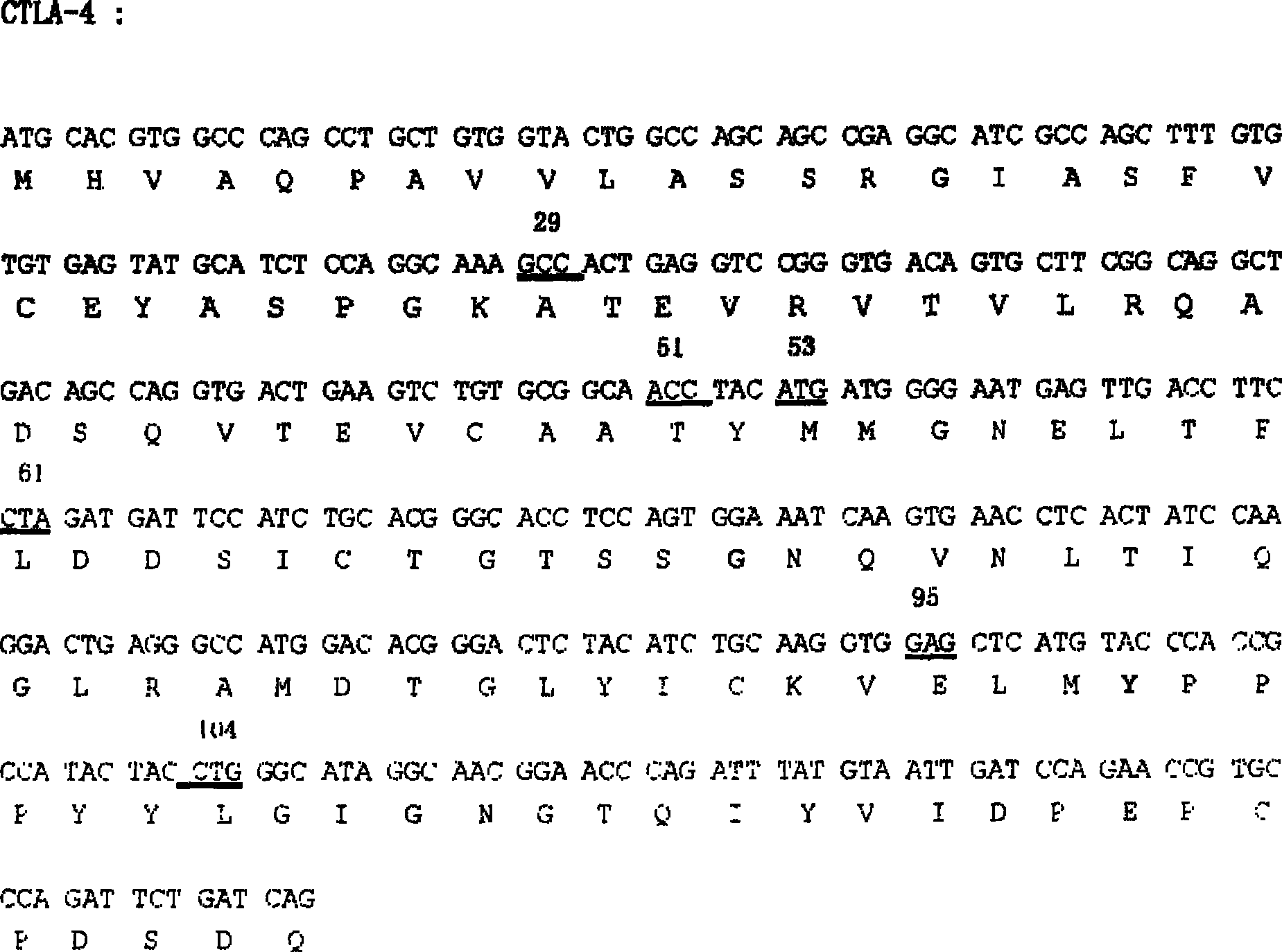
[0032] Example 1. Cloning of CTLA-4 extramembrane region gene and Fc region gene
[0033] Lymphocytes from healthy people were separated with lymphocyte separation medium, total RNA was extracted with Trizol reagent (product of Invitrogen Company), primers were designed to amplify the CTLA-4 extramembrane region gene (GeneID: 1493), and primers FC sense: GCCCAGATTCTGATCAGGAGCCCAAATCTTCTGAC and FC reverse Sense: GAATTCTCATTTACCCGGAGACAGG amplifies the antibody Fc region. All PCR reactions were hot-started, and the reaction conditions were: 94°C for 45 seconds, 60°C for 45 seconds, 72°C for 1 minute and 10 seconds, 30 cycles; 72°C for 10 minutes. The PCR product was purified and recovered by agarose gel electrophoresis and cloned into the pGEM-T (promega company) vector. After sequencing verification, it was confirmed that the correct clone was obtained. SEQ ID NO: 1 and SEQ ID NO: 2 show the nucleotide and amino acid sequences of the extramembrane region of CTLA-4. SEQ ID NO:...
Embodiment 2
[0034] The expression vector construction of embodiment 2.CTLA4-Ig fusion protein
[0035] Design primers to perform overlapPCR on the synthesized signal peptide sequence SEQ ID NO: 5 and the cloned CTLA-4 extramembrane gene fragment, perform overlapPCR on the sequenced correct fragment and antibody IgG1 Fc, and install pGEM-T vector for sequencing. Select the clone with correct sequencing and cut the CTLA4-Ig fusion protein gene with HindIII and EcoR I, purify and recover through agarose gel electrophoresis, and use HindIII and EcoR I to digest the plasmid pcDNA3. The connection was carried out to construct the eukaryotic expression vector pcDNA3.1(+), denoted as pcDNA3.1(+)(CTLA4-Ig).
Embodiment 3
[0036] Example 3. Stable expression and purification of fusion protein
[0037] Inoculate 3×10 in a 3.5cm tissue culture dish 5 CHO-K1 cells (ATCC CRL-9618), transfected when the cells were cultured to 90%-95% confluence: get 10 μg of plasmid (plasmid pcDNA3.1 (+) (CTLA4-Ig) 10 μg and 20 μl of Lipofectamine2000Reagent (product of Invitrogen Company) respectively Dissolve in 500μl serum-free DMEM medium, let stand at room temperature for 5 minutes, mix the above two liquids, incubate at room temperature for 20 minutes to form DNA-liposome complexes, and replace the culture dish with 3ml serum-free DMEM medium serum-containing medium, and then add the formed DNA-liposome complexes to the plate, CO 2 After culturing in the incubator for 4 hours, add 2ml of DMEM complete medium containing 10% serum and place in CO 2 Continue to grow in the incubator. After 24 hours of transfection, the cells were replaced with selection medium containing 600 μg / ml G418 to screen for resistant c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com