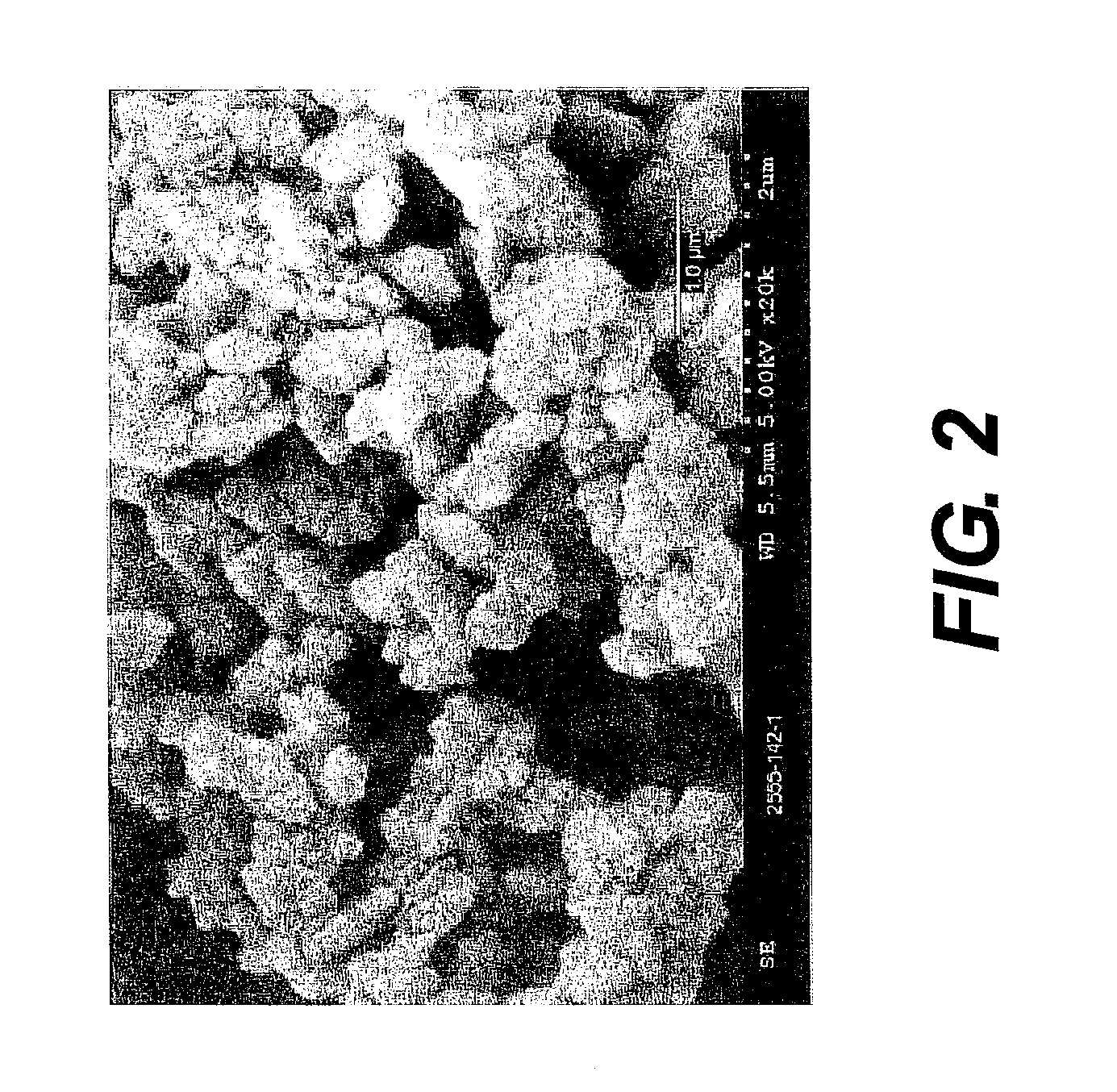
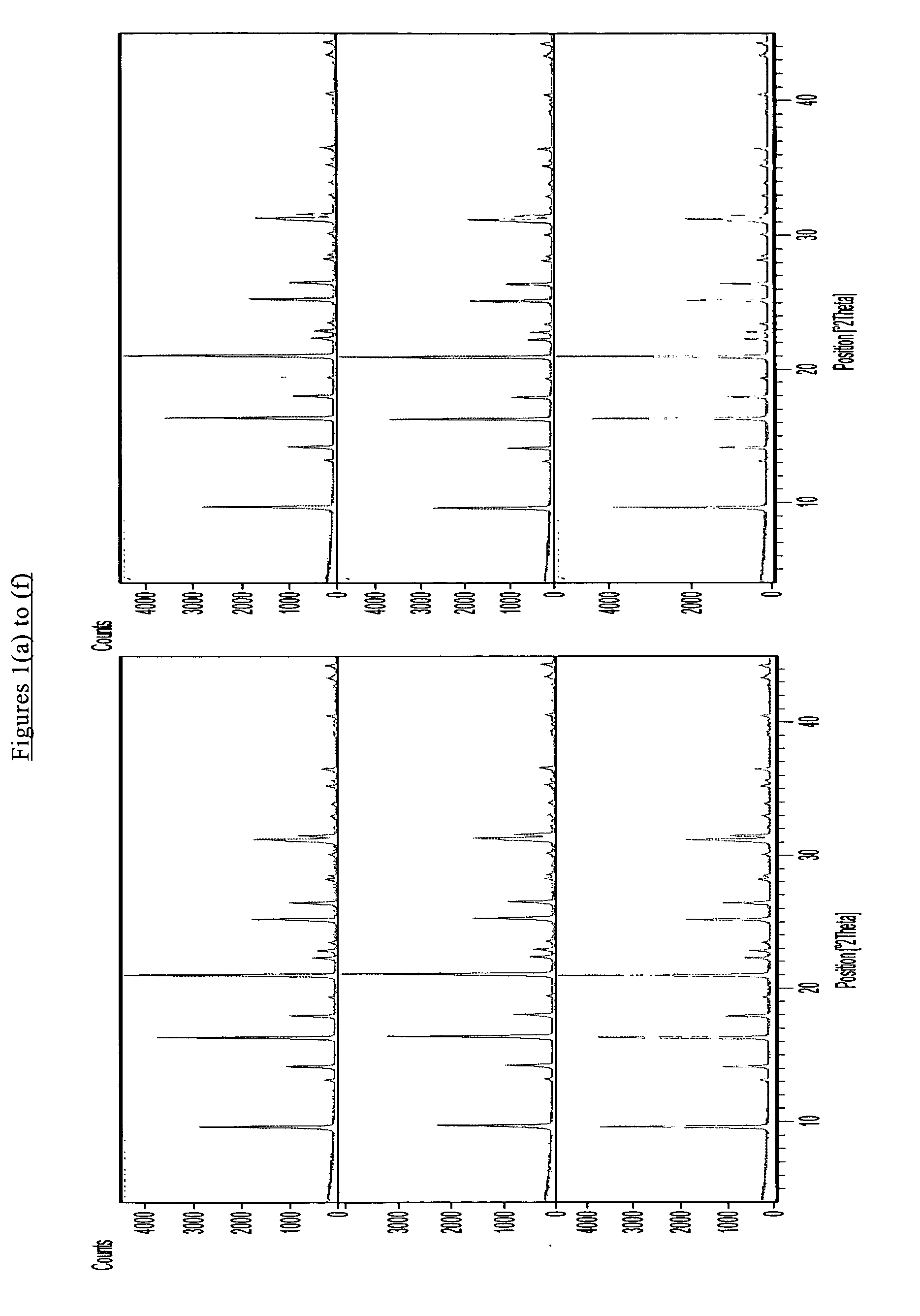
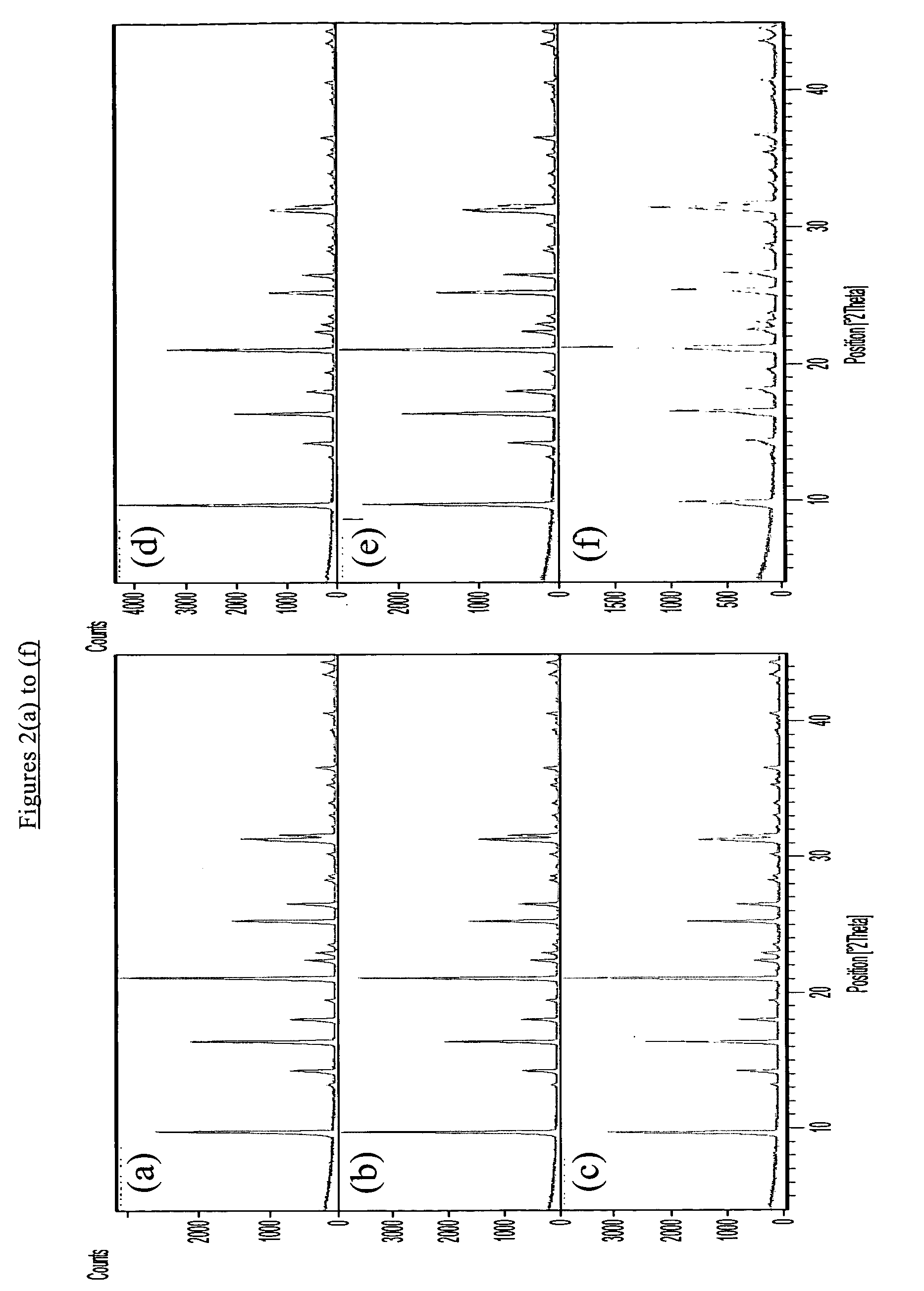
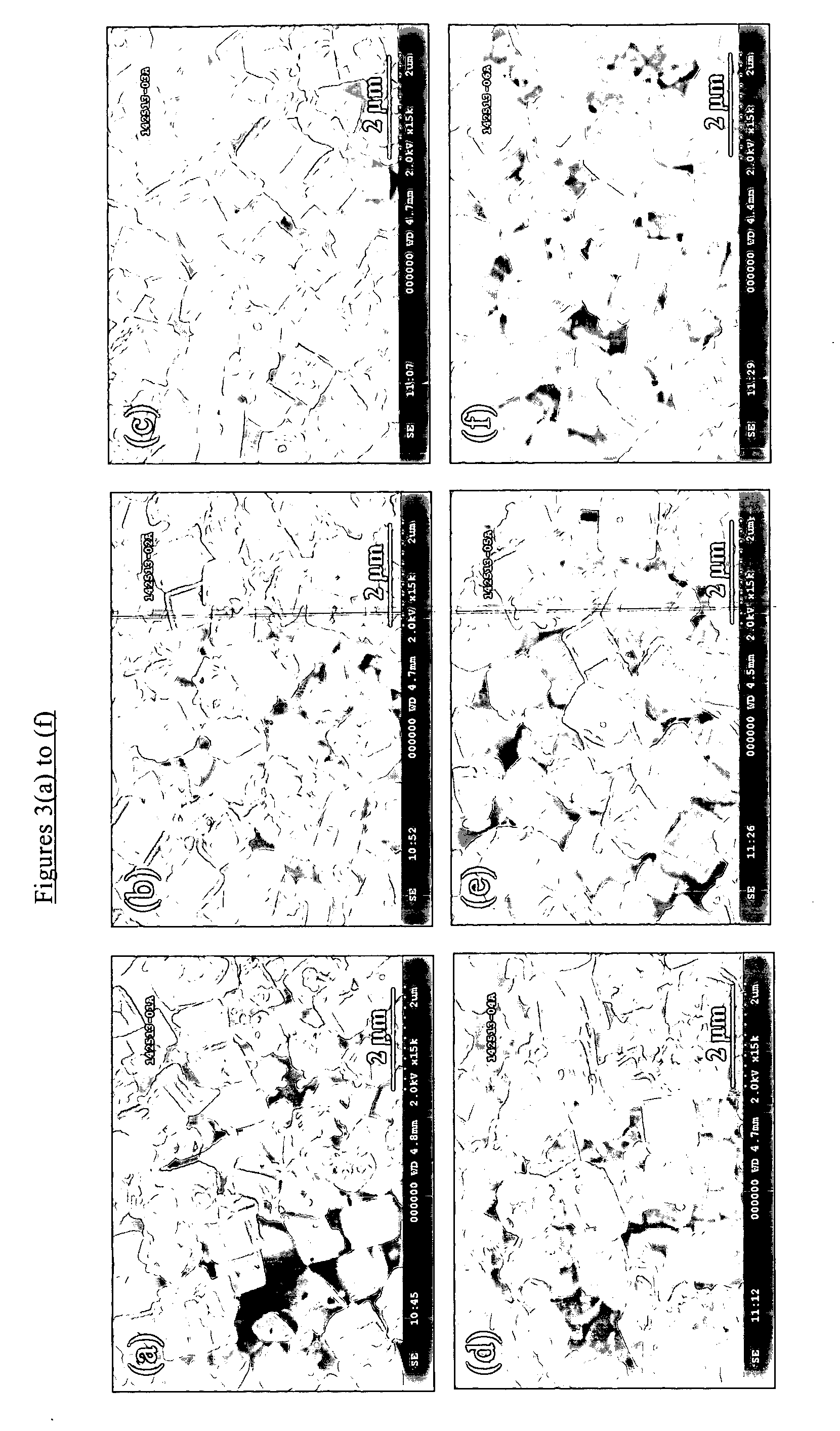
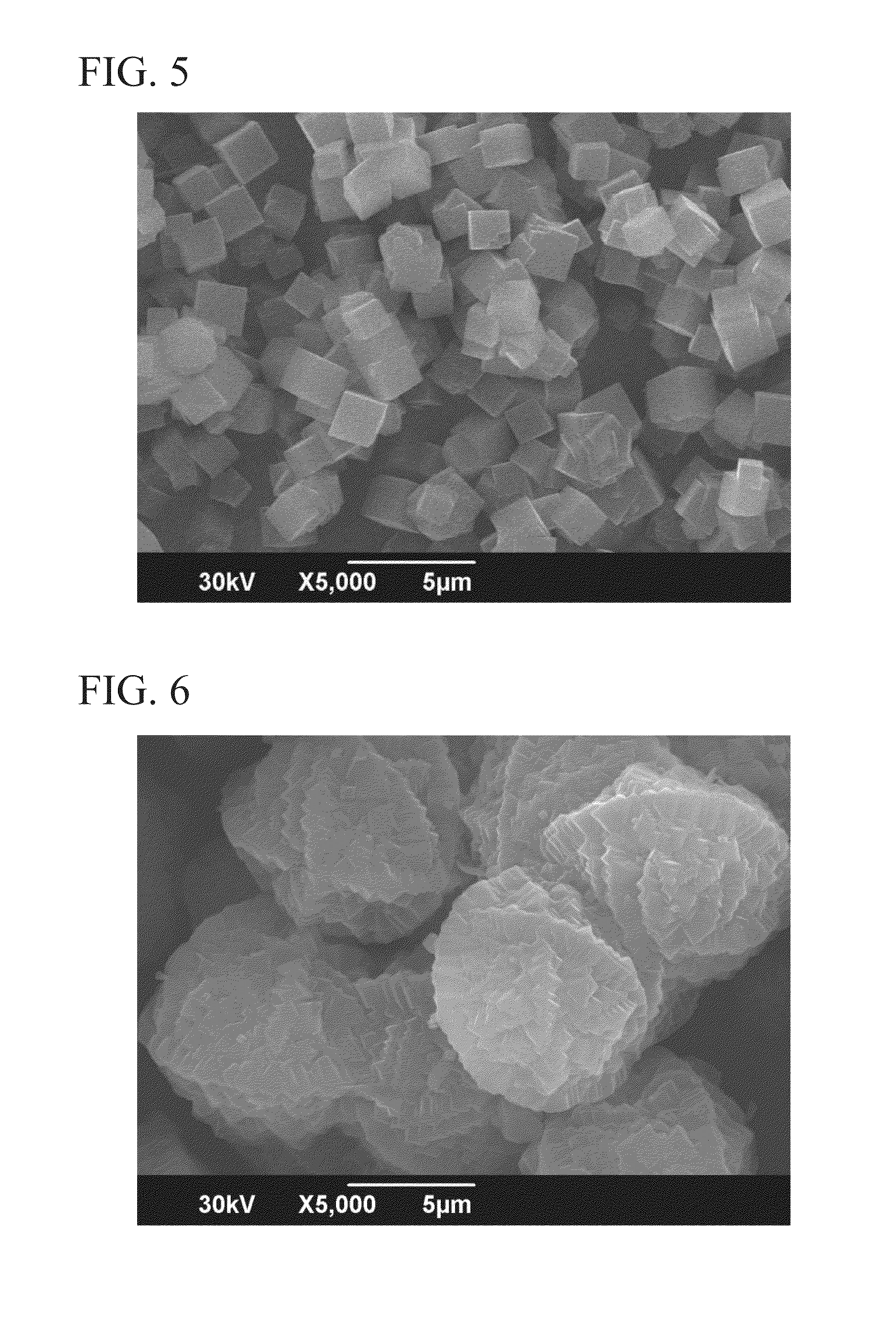
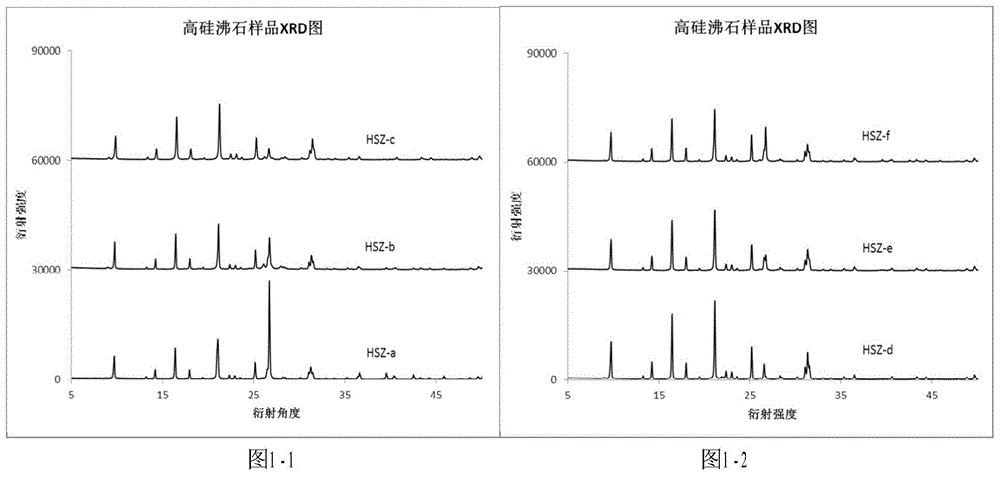
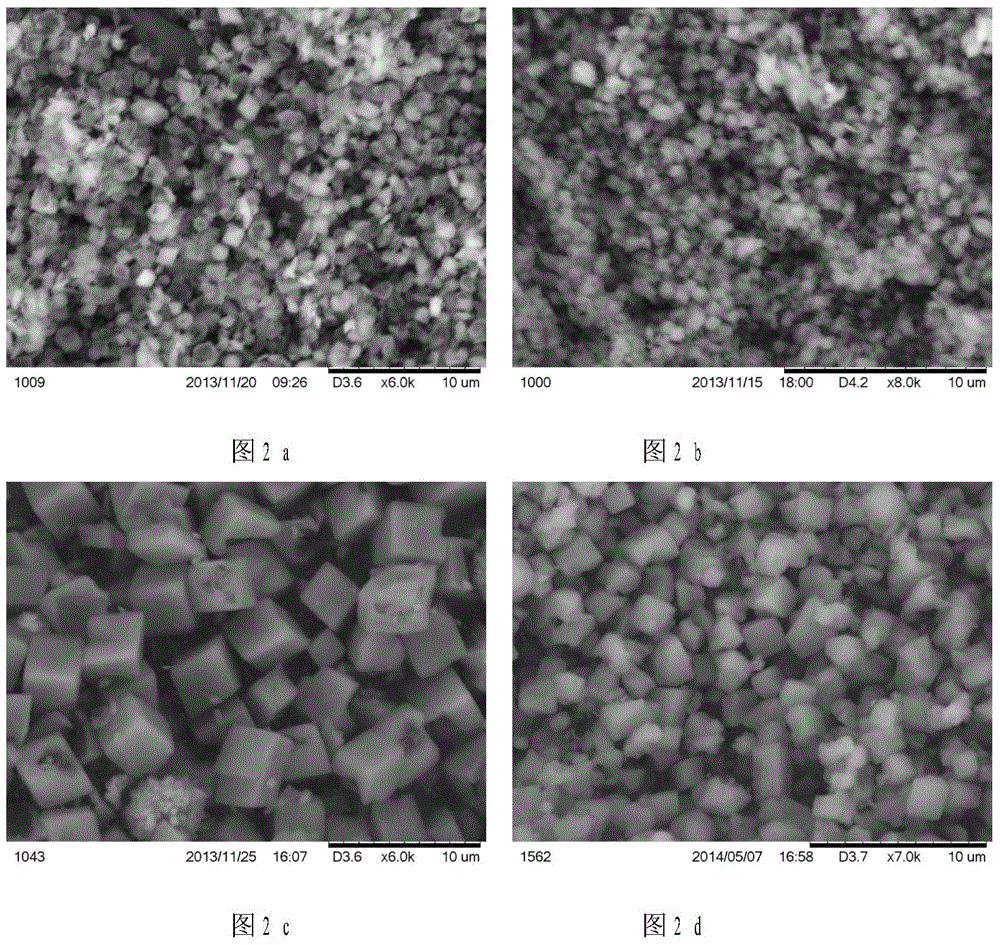
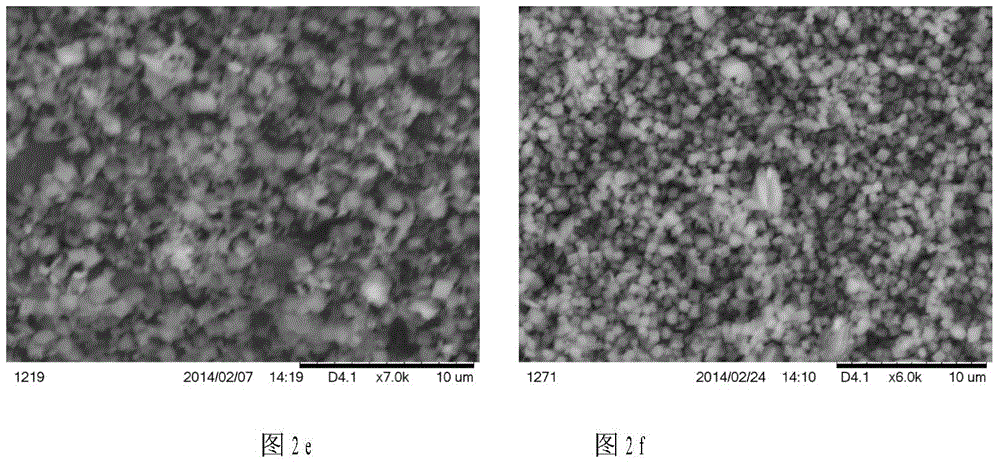
Patents
Literature
145 results about "Chabazite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
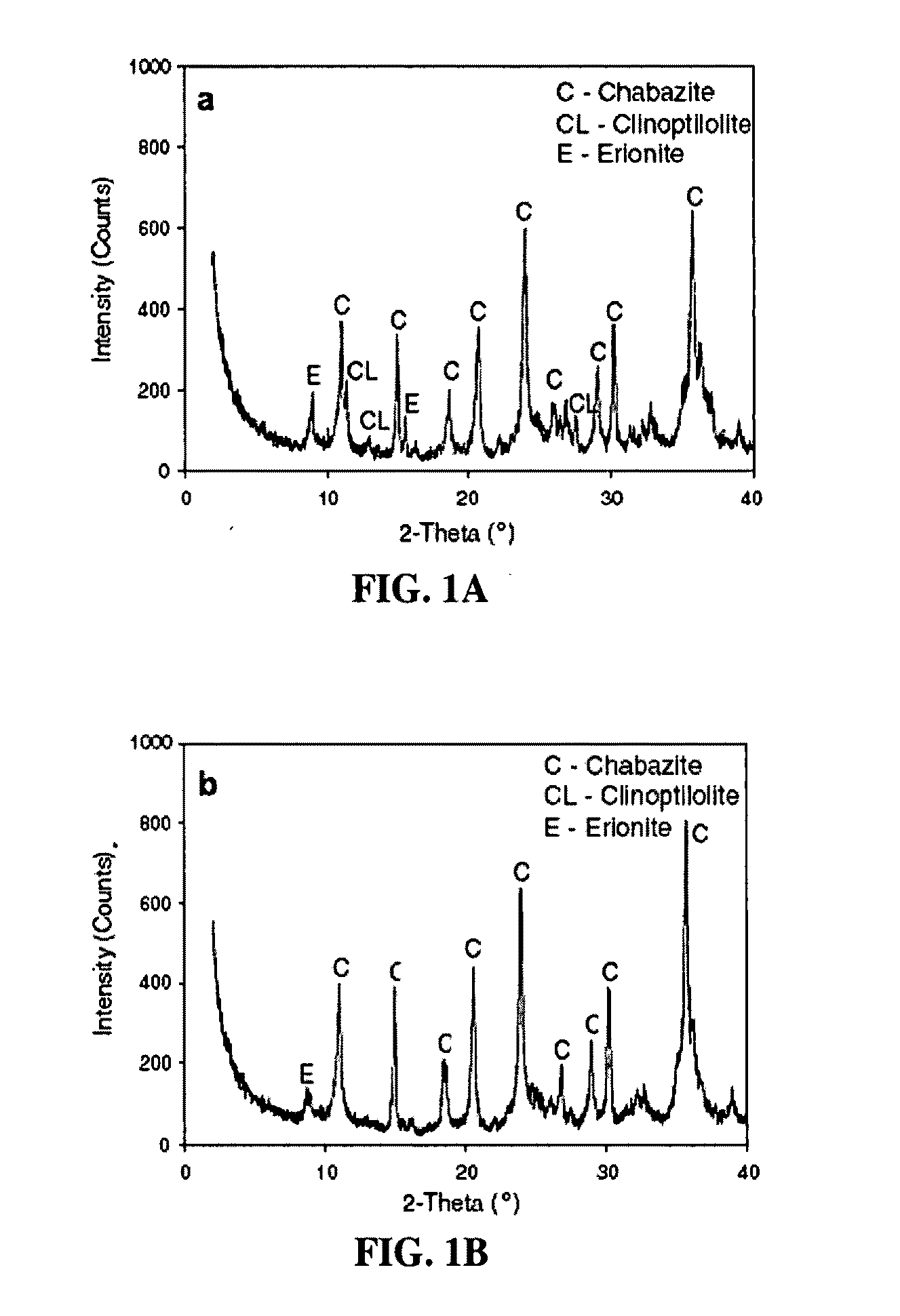
Chabazite (UK: /ˈkæbəzaɪt/) is a tectosilicate mineral of the zeolite group, closely related to gmelinite, with formula (Ca,Na₂,K₂,Mg)Al₂Si₄O₁₂·6H₂O. Recognized varieties include Chabazite-Ca, Chabazite-K, Chabazite-Na, and Chabazite-Sr, depending on the prominence of the indicated cation.
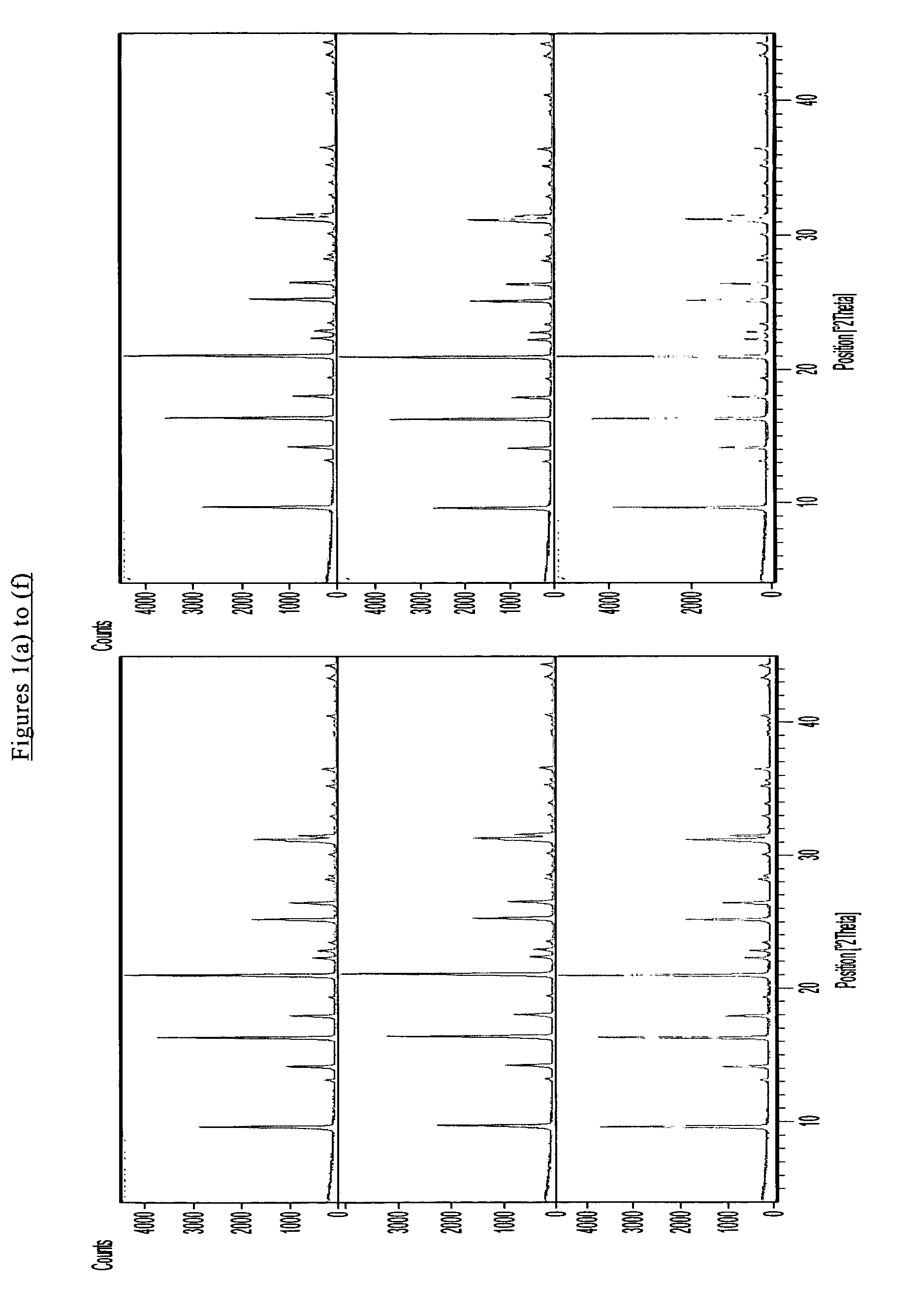
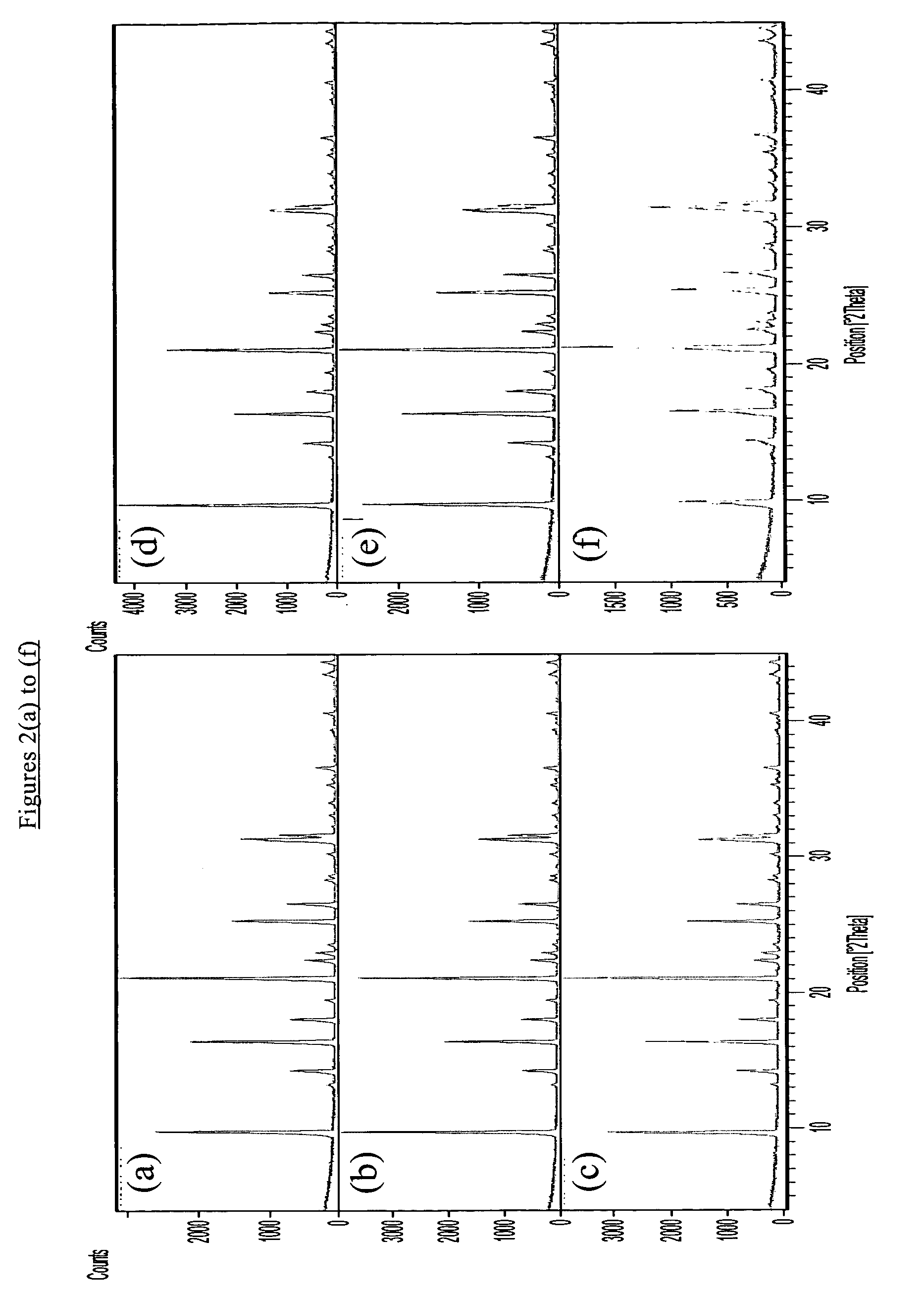
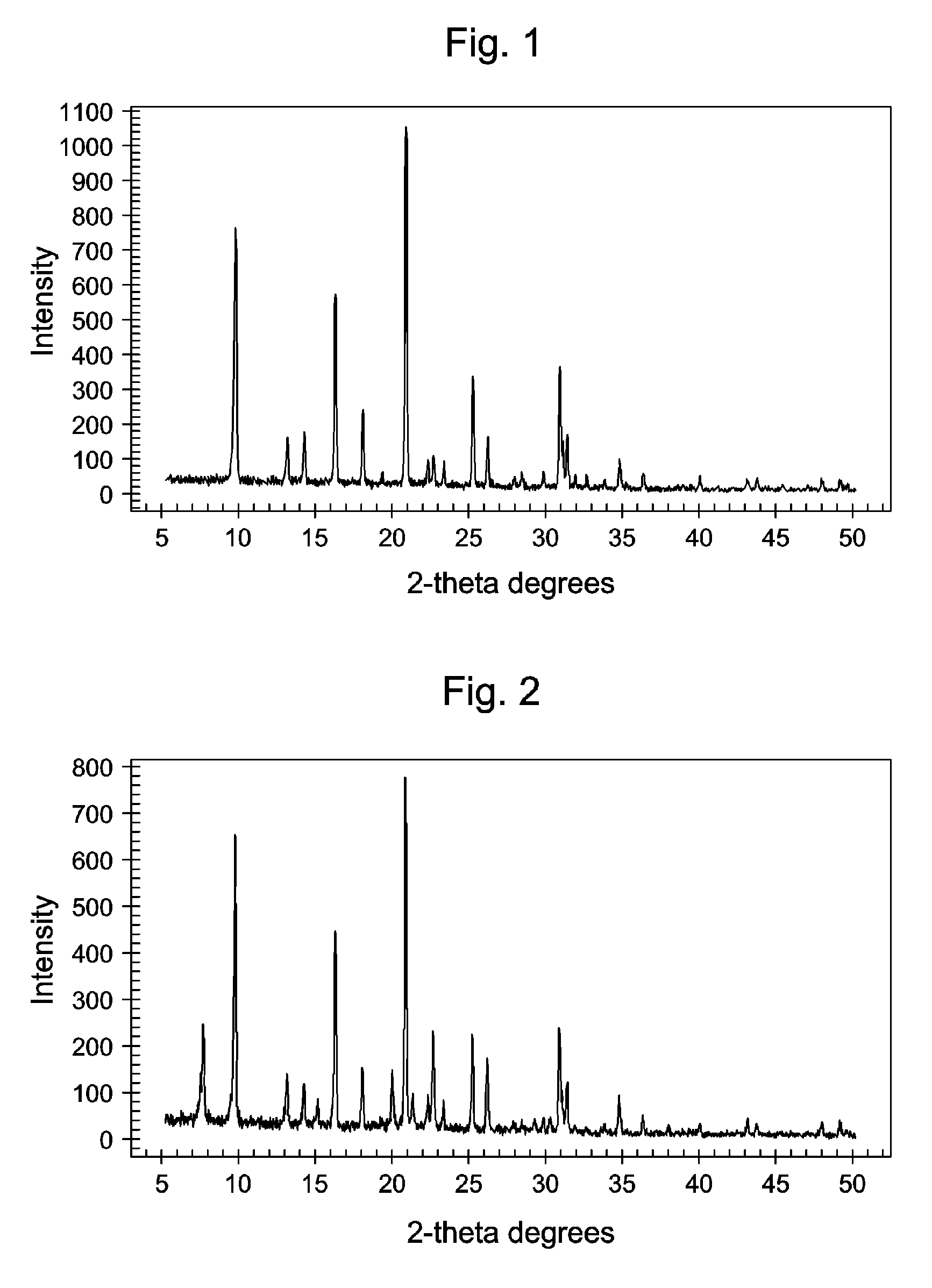
Chabazite-containing molecular sieve, its synthesis and its use in the conversion of oxygenates to olefins
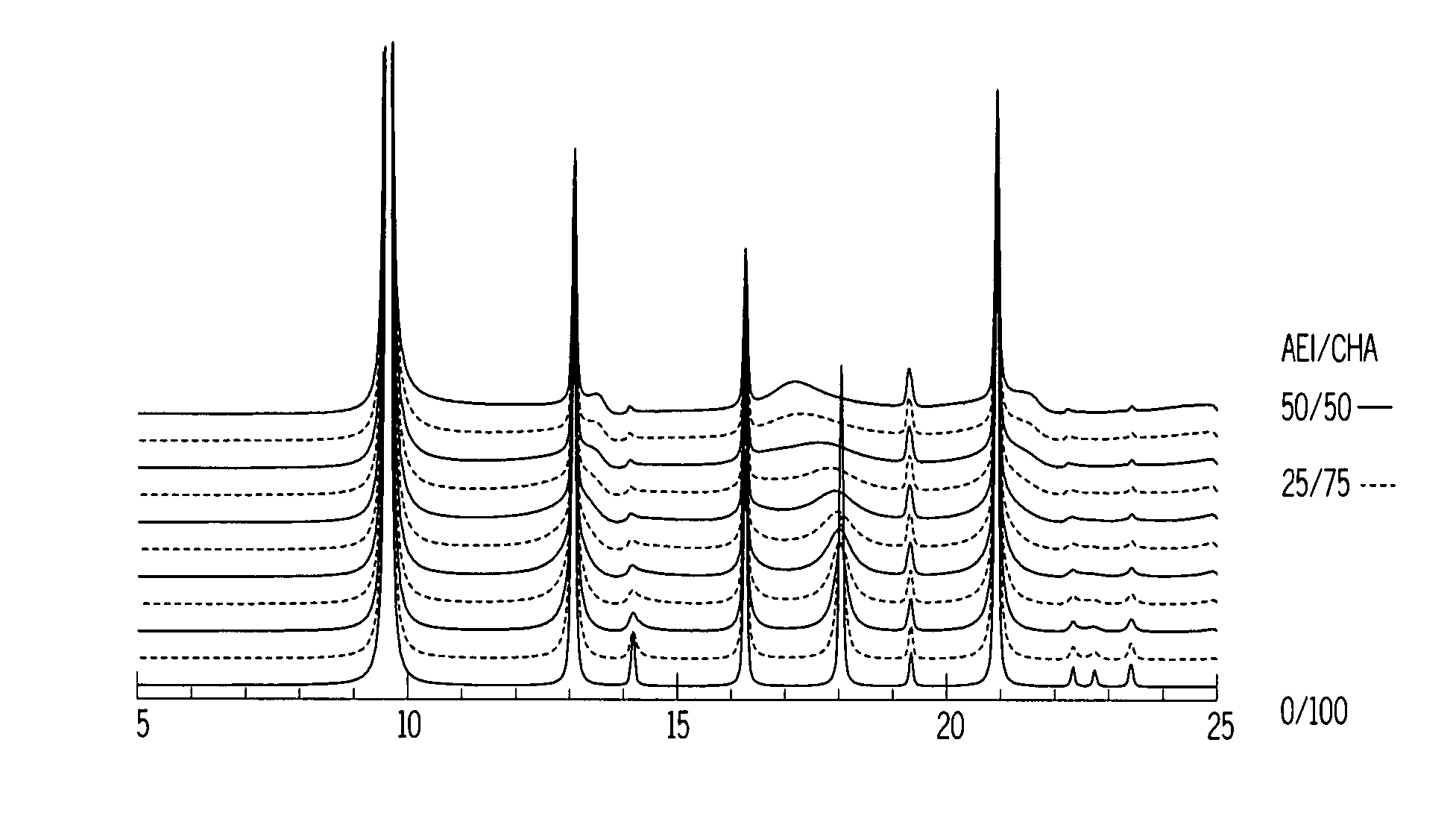
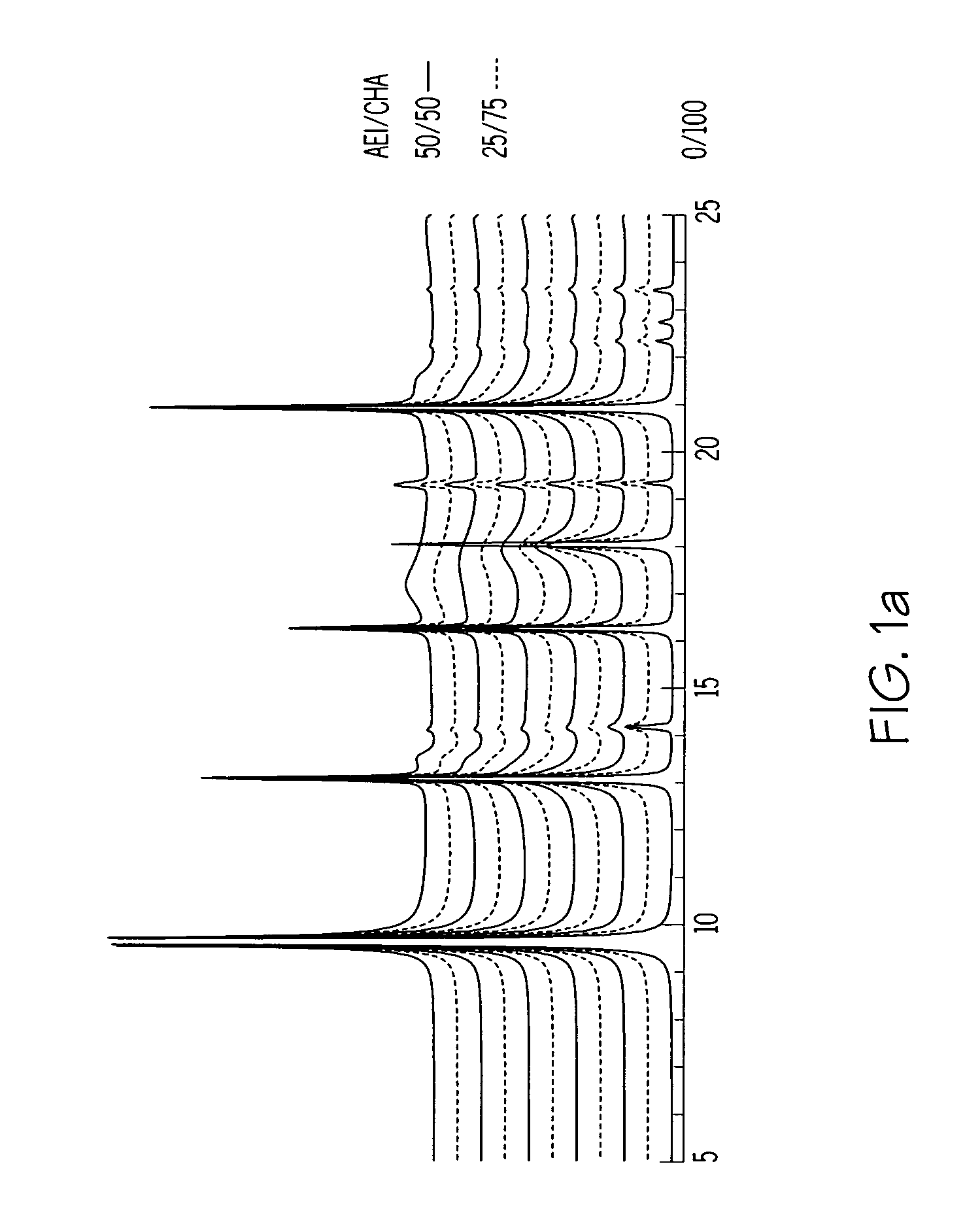
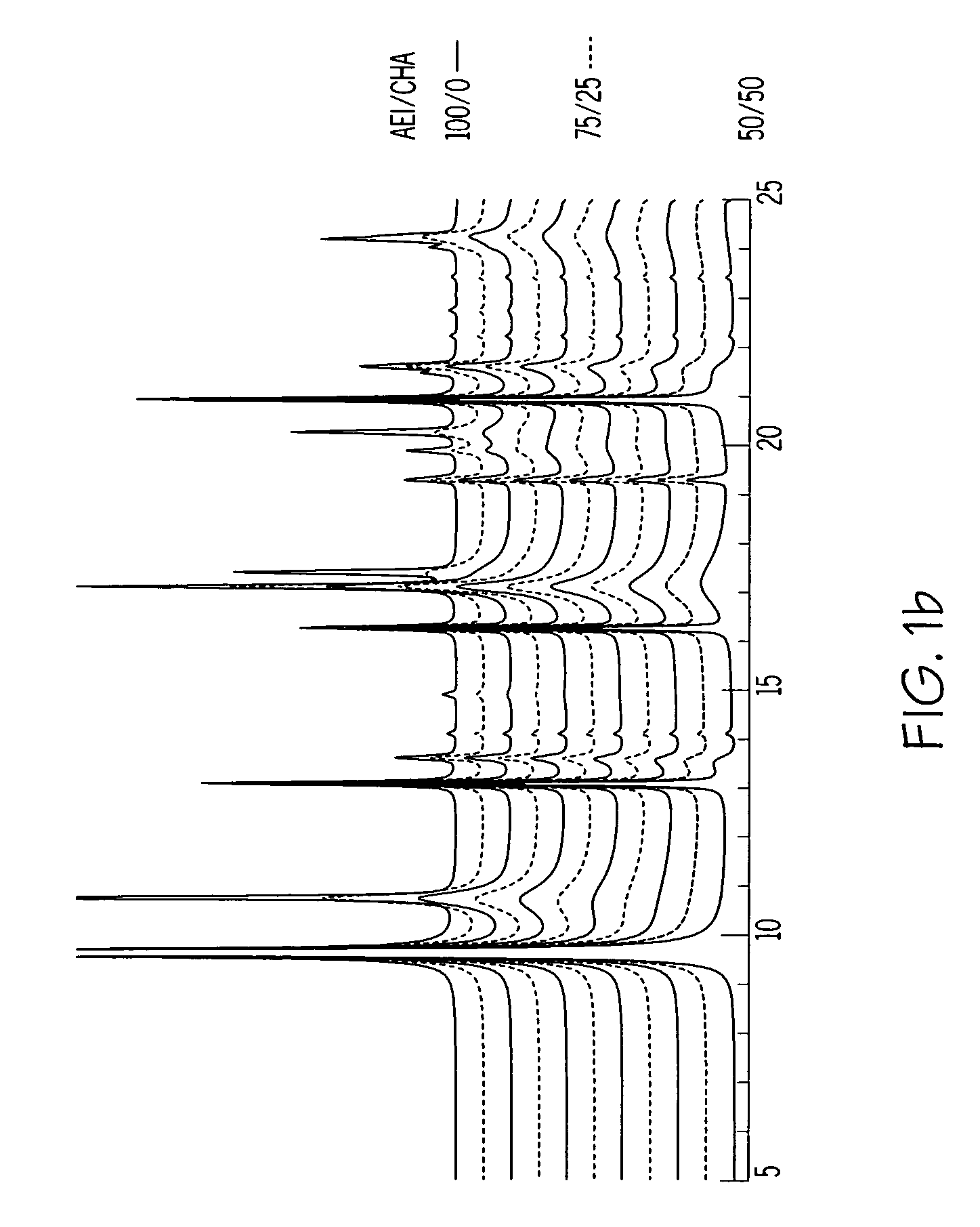
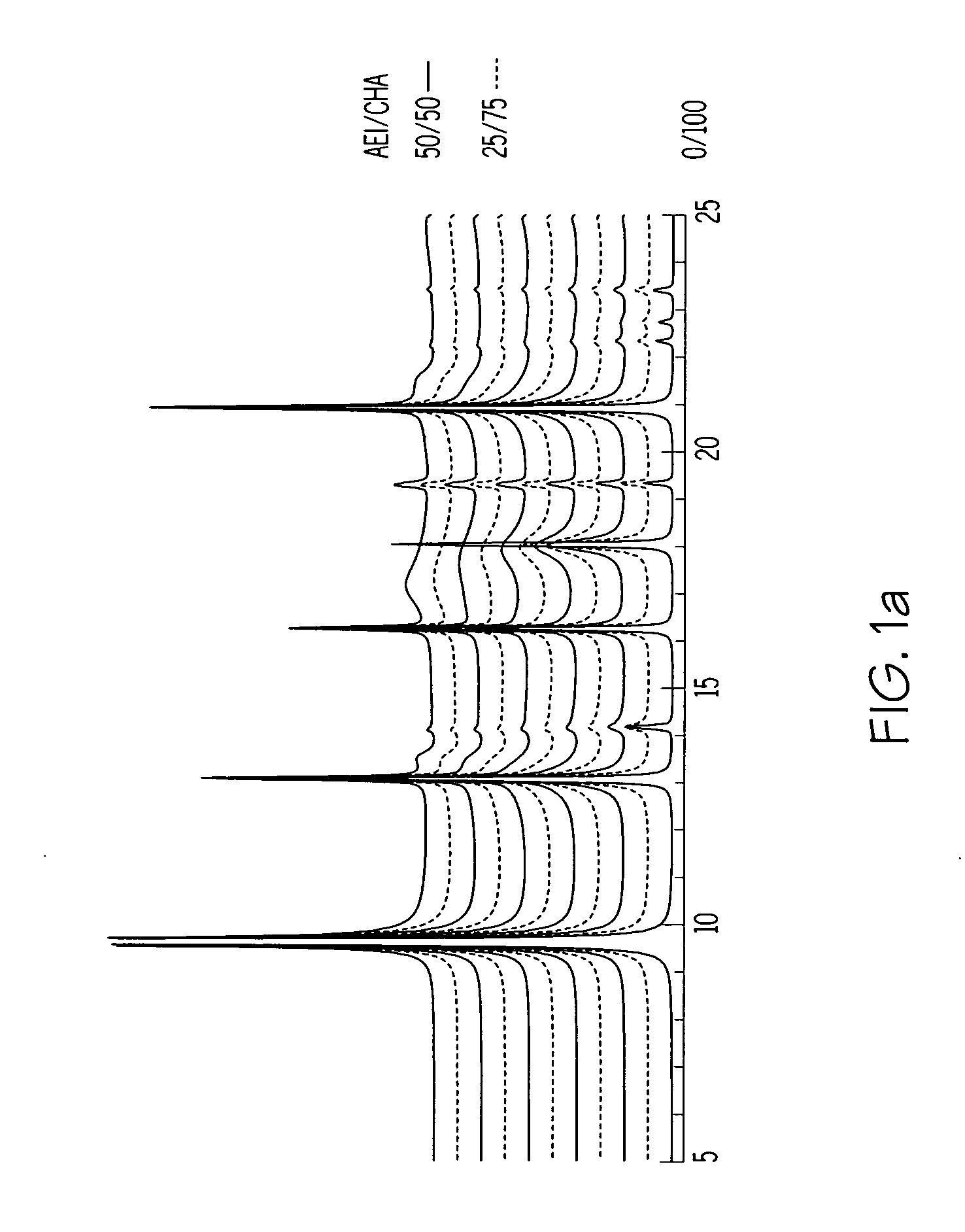
A crystalline material substantially free of framework phosphorus and comprising a CHA framework type molecular sieve with stacking faults or at least one intergrown phase of a CHA framework type molecular sieve and an AEI framework type molecular sieve, wherein said material, in its calcined, anhydrous form, has a composition involving the molar relationship:(n)X2O3:YO2,wherein X is a trivalent element; Y is a tetravalent element; and n is from 0 to about 0.5. The material exhibits activity and selectivity in the conversion of methanol to lower olefins, especially ethylene and propylene.
Owner:EXXONMOBIL CHEM PAT INC +1
Synthesis of chabazite-containing molecular sieves and their use in the conversion of oxygenates to olefins
The synthesis of a crystalline material, in particular, a high silica zeolite, comprising a chabazite-type framework molecular sieve is conducted in the presence of an organic directing agent having the formula: [R1R2R3N—R4]+Q−wherein R1 and R2 are independently selected from hydrocarbyl groups and hydroxy-substituted hydrocarbyl groups having from 1 to 3 carbon atoms, provided that R1 and R2 may be joined to form a nitrogen-containing heterocyclic structure, R3 is an alkyl group having 2 to 4 carbon atoms and R4 is selected from a 4- to 8-membered cycloalkyl group, optionally, substituted by 1 to 3 alkyl groups each having from 1 to 3 carbon atoms; and a 4- to 8-membered heterocyclic group having from 1 to 3 heteroatoms, said heterocyclic group being, optionally, substituted by 1 to 3 alkyl groups each having from 1 to 3 carbon atoms and the or each heteroatom in said heterocyclic group being selected from the group consisting of O, N, and S, or R3 and R4 are hydrocarbyl groups having from 1 to 3 carbon atoms joined to form a nitrogen-containing heterocyclic structure; and Q− is a anion.
Owner:EXXONMOBIL CHEM PAT INC
Synthesis of molecular sieves having the chabazite framework type and their use in the conversion of oxygenates to olefins
The synthesis of a crystalline aluminophosphate or silicoaluminophosphate molecular sieve having a chabazite-type framework type is conducted in the presence of an organic directing agent having the formula (I) [R1R2R3N—R4]+X− (I) wherein R1, R2 and R3 are independently selected from the group consisting of alkyl groups having from 1 to 3 carbon atoms and hydroxyalkyl groups having from 1 to 3 carbon atoms; R4 is selected from the group consisting of 4- to 8-membered cycloalkyl groups, optionally substituted by 1 to 3 alkyl groups having from 1 to 3 carbon atoms; 4- to 8-membered heterocyclic groups having from 1 to 3 heteroatoms, said heterocyclic groups being optionally substituted by 1 to 3 alkyl groups having from 1 to 3 carbon atoms and the heteroatoms in said heterocyclic groups being selected from the group consisting of O, N, and S; and aromatic groups optionally substituted by 1 to 3 alkyl groups, said alkyl groups having from 1 to 3 carbon atoms; and X− is an anion.
Owner:EXXONMOBIL CHEM PAT INC
Cementing compositions containing substantially spherical zeolite
ActiveUS20050133222A1Improve flexural strengthReduce brittlenessDrilling compositionSealing/packingPortland cementNatrolite
A cementitious composition for cementing an oil or gas well and which exhibits, when cured, increased flexural strength and a flexural strength to compressive strength ratio between from about 0.29 to about 0.80, contains a hydraulically-active cementitious material, such as Portland cement, and substantially spherical zeolite. Representative zeolites include natrolite, heulandite, analcime, chabazite, stilbite, and clinoptilolite. The weight percent of zeolite in the cement composition is generally less than or equal to 15 percent. In practice, a well bore may be cemented by pumping the activated slurry and pumping it within the well bore to a pre-selected location and allowing it to solidify.
Owner:BAKER HUGHES INC
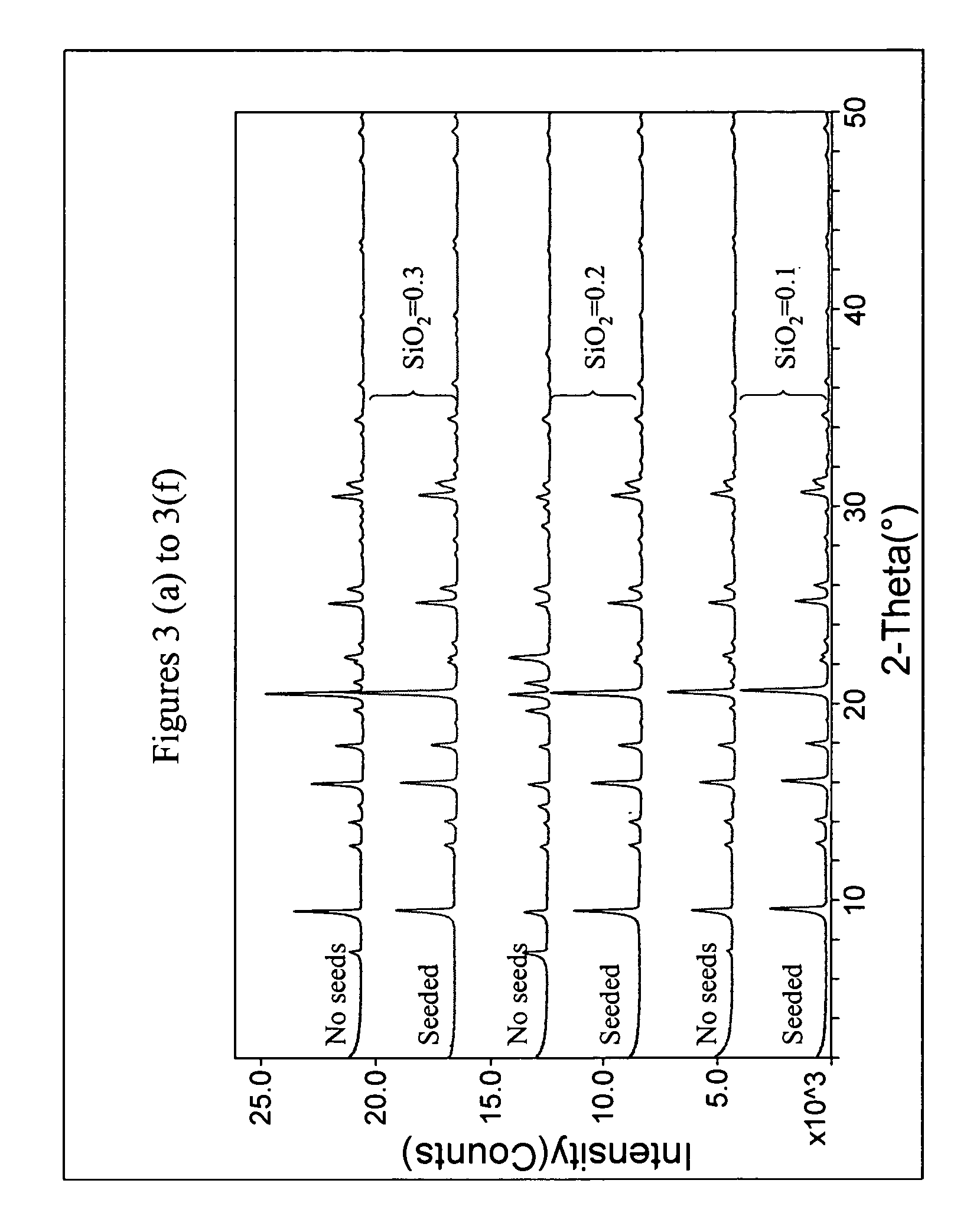
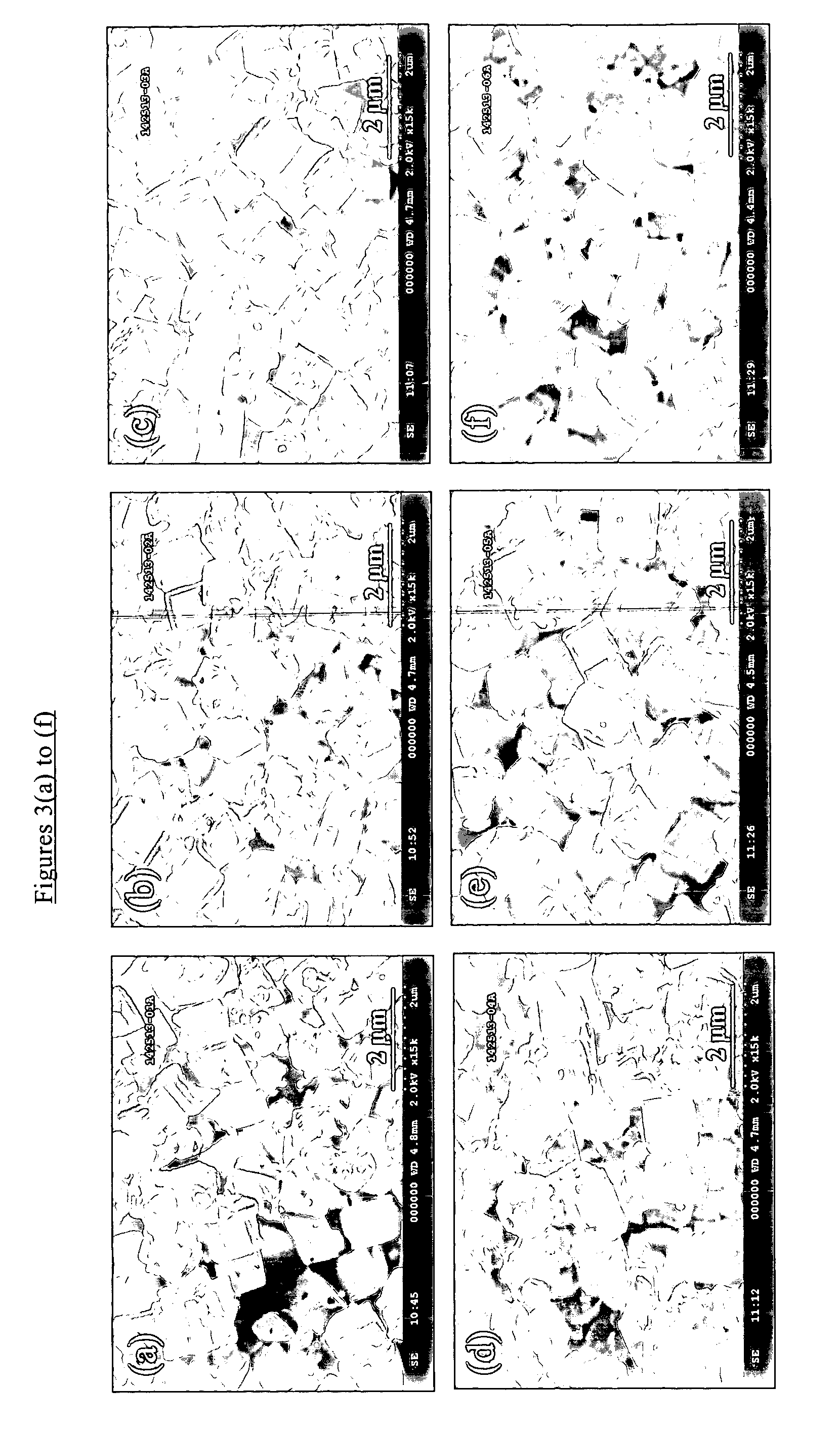
Chabazite-type molecular sieve, its synthesis and its use in the conversion of oxygenates to olefins
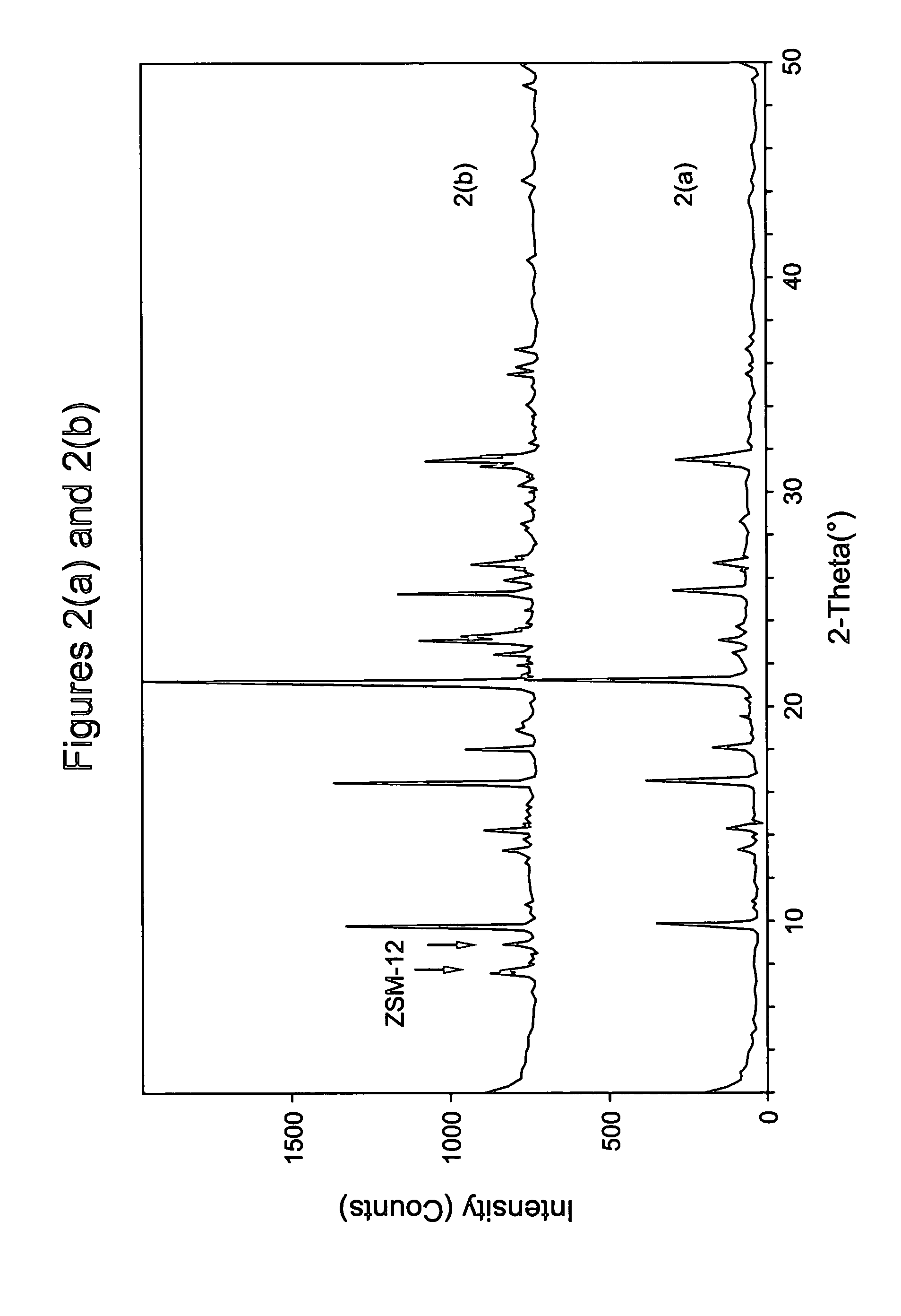
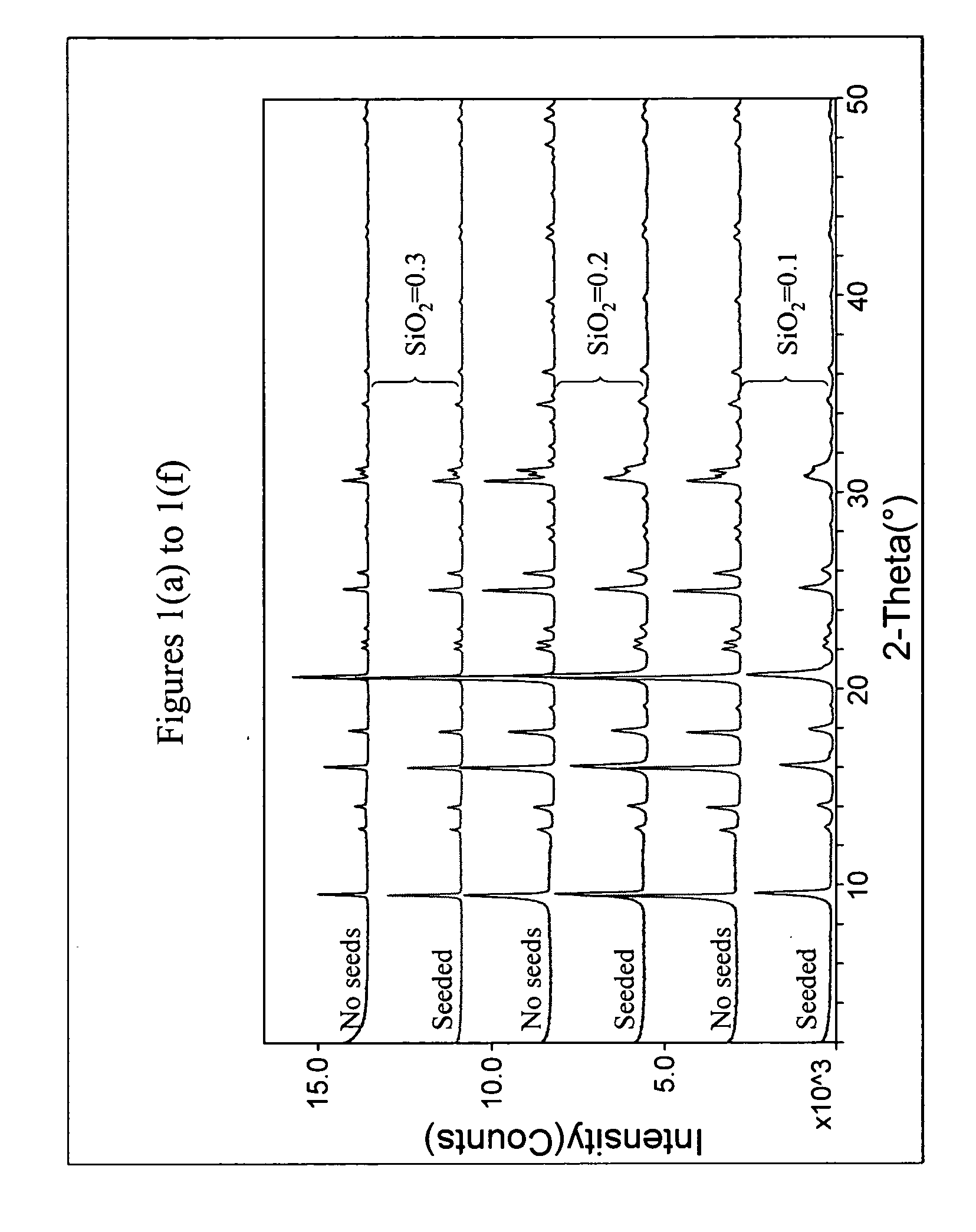
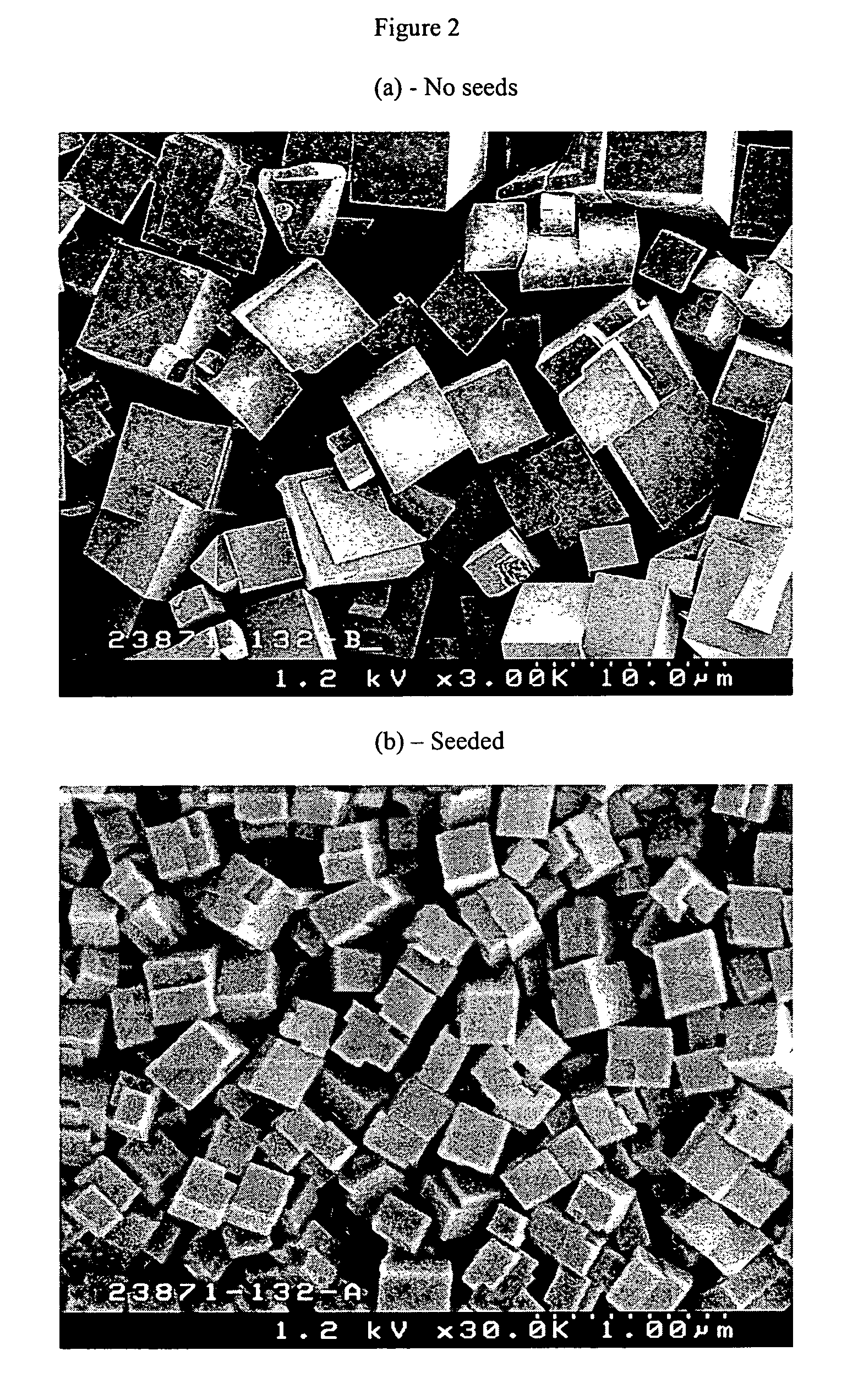
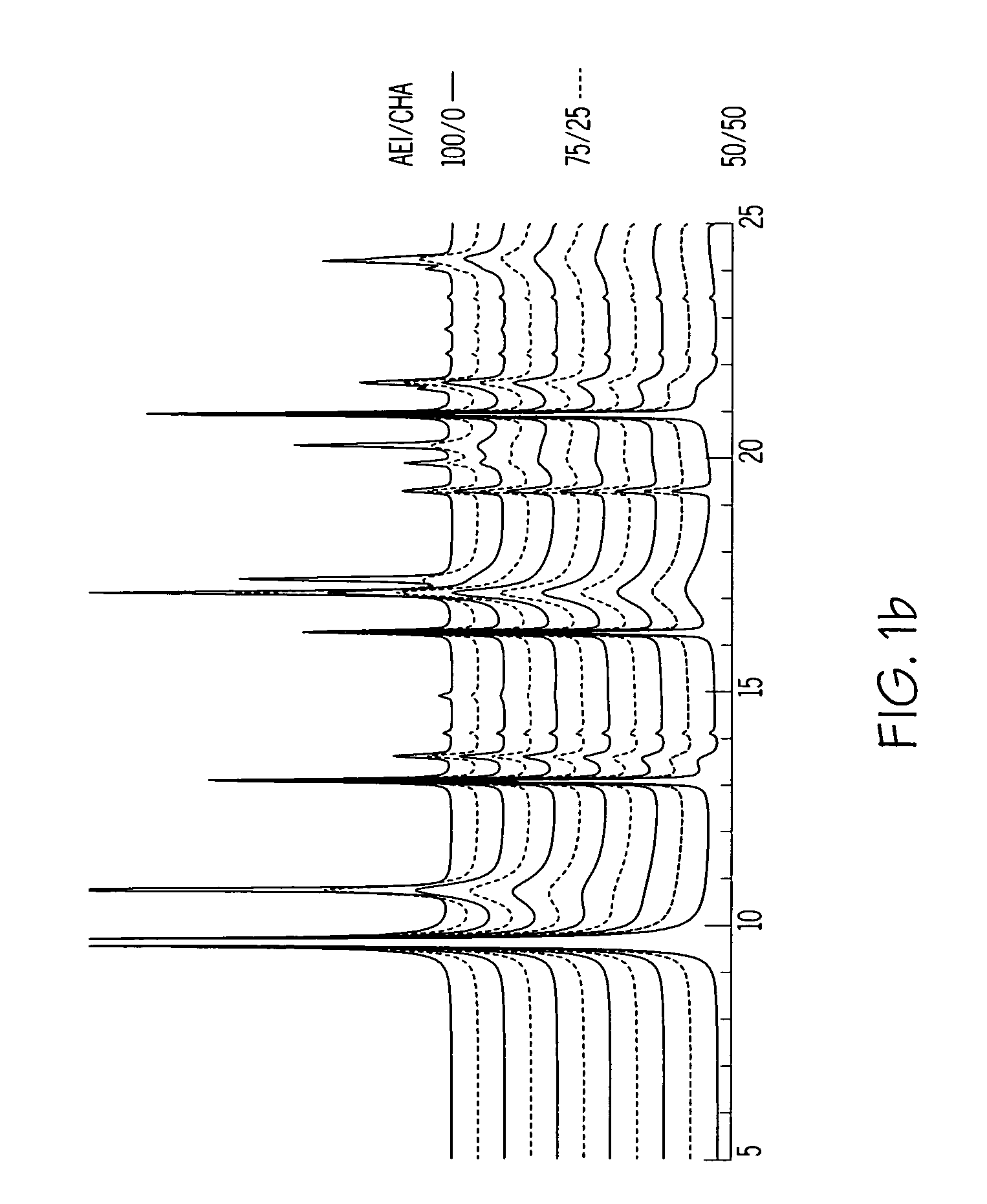
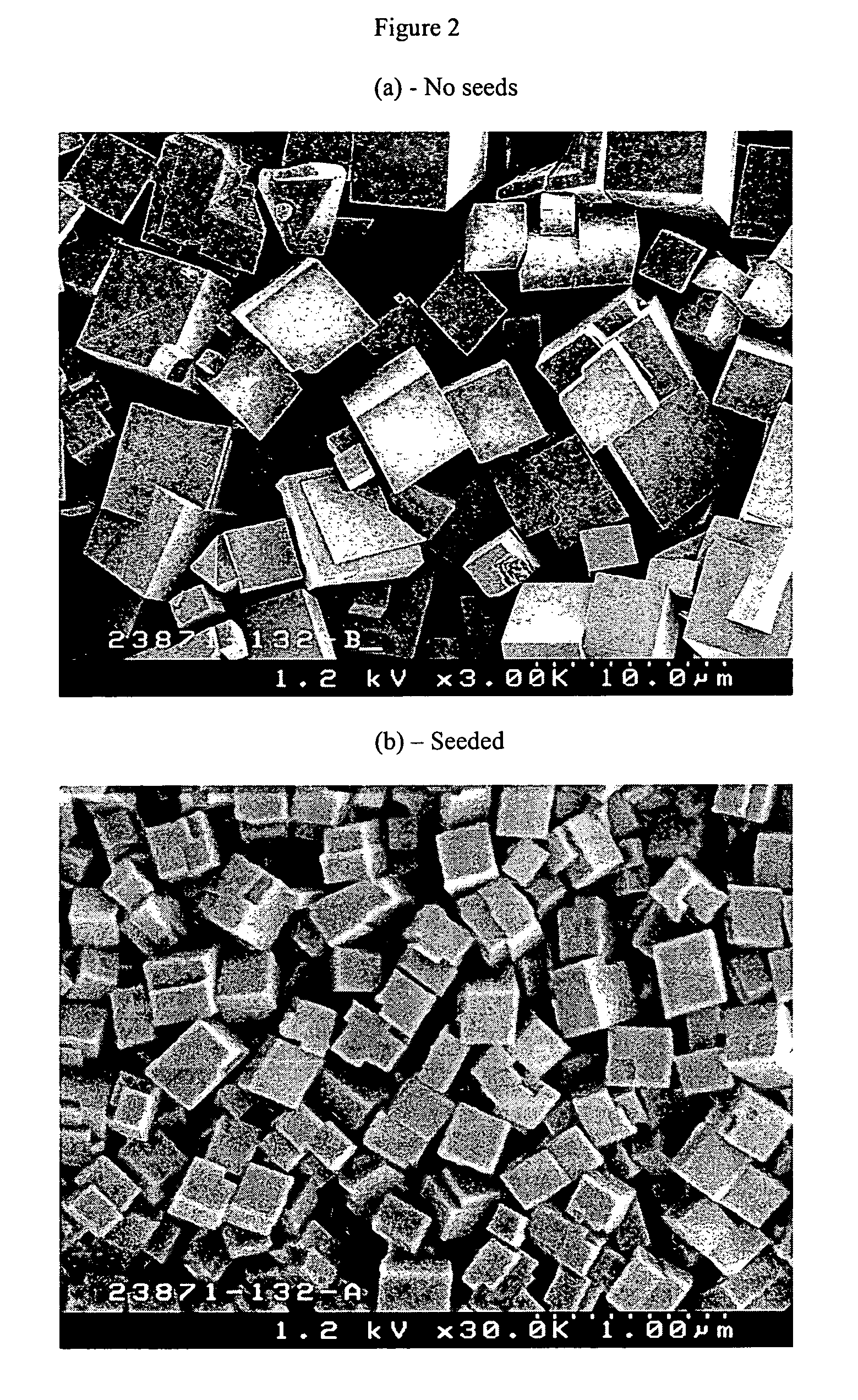
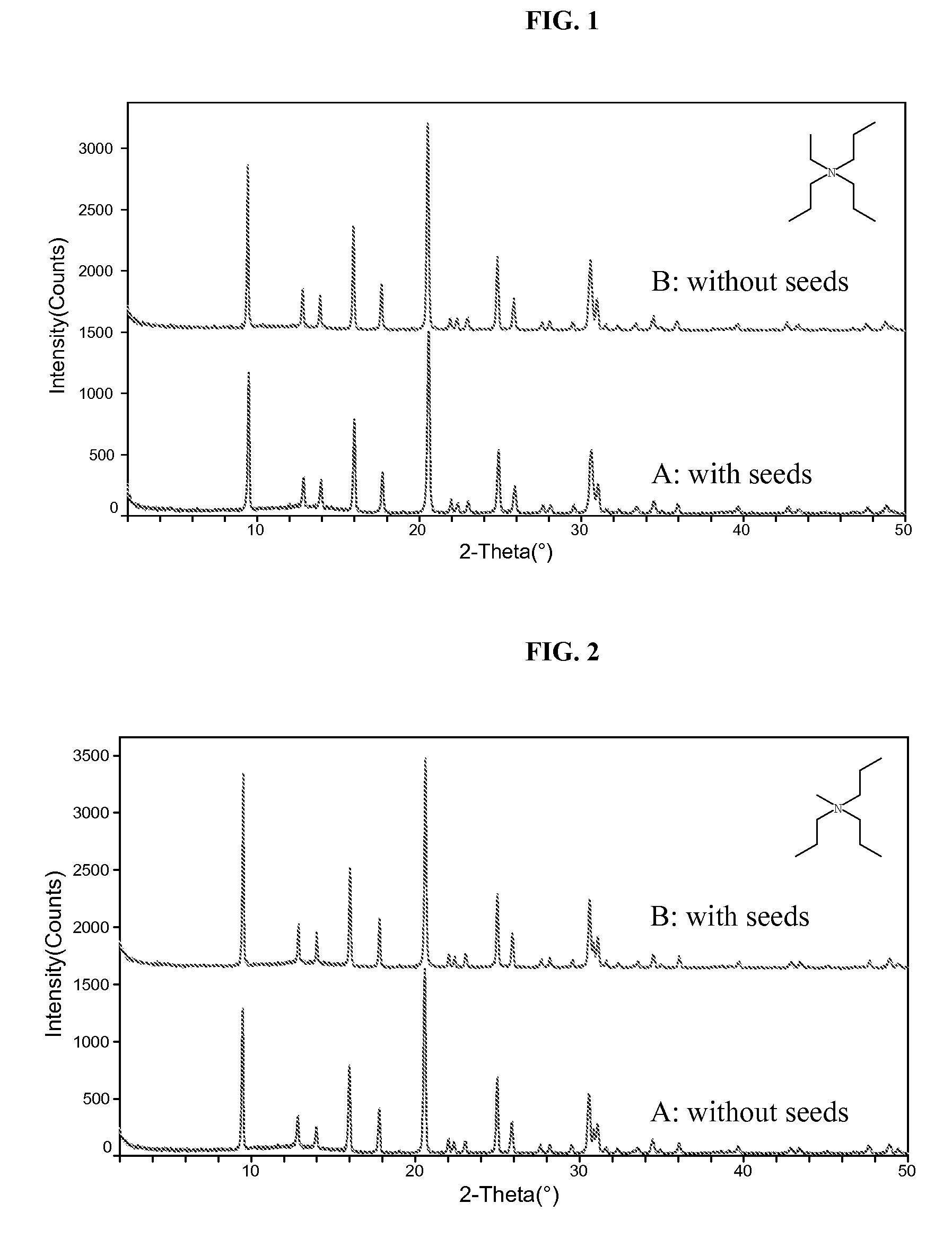
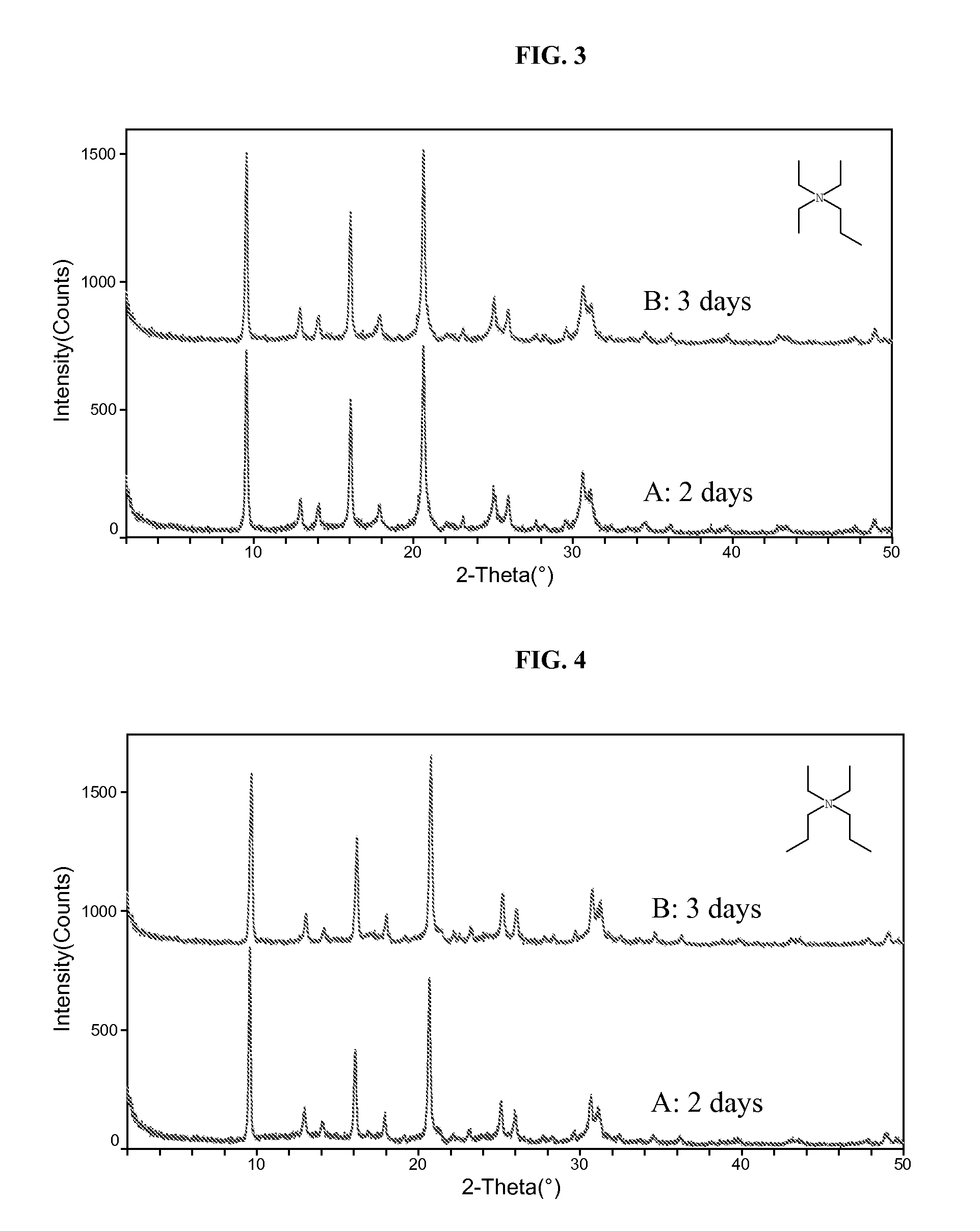
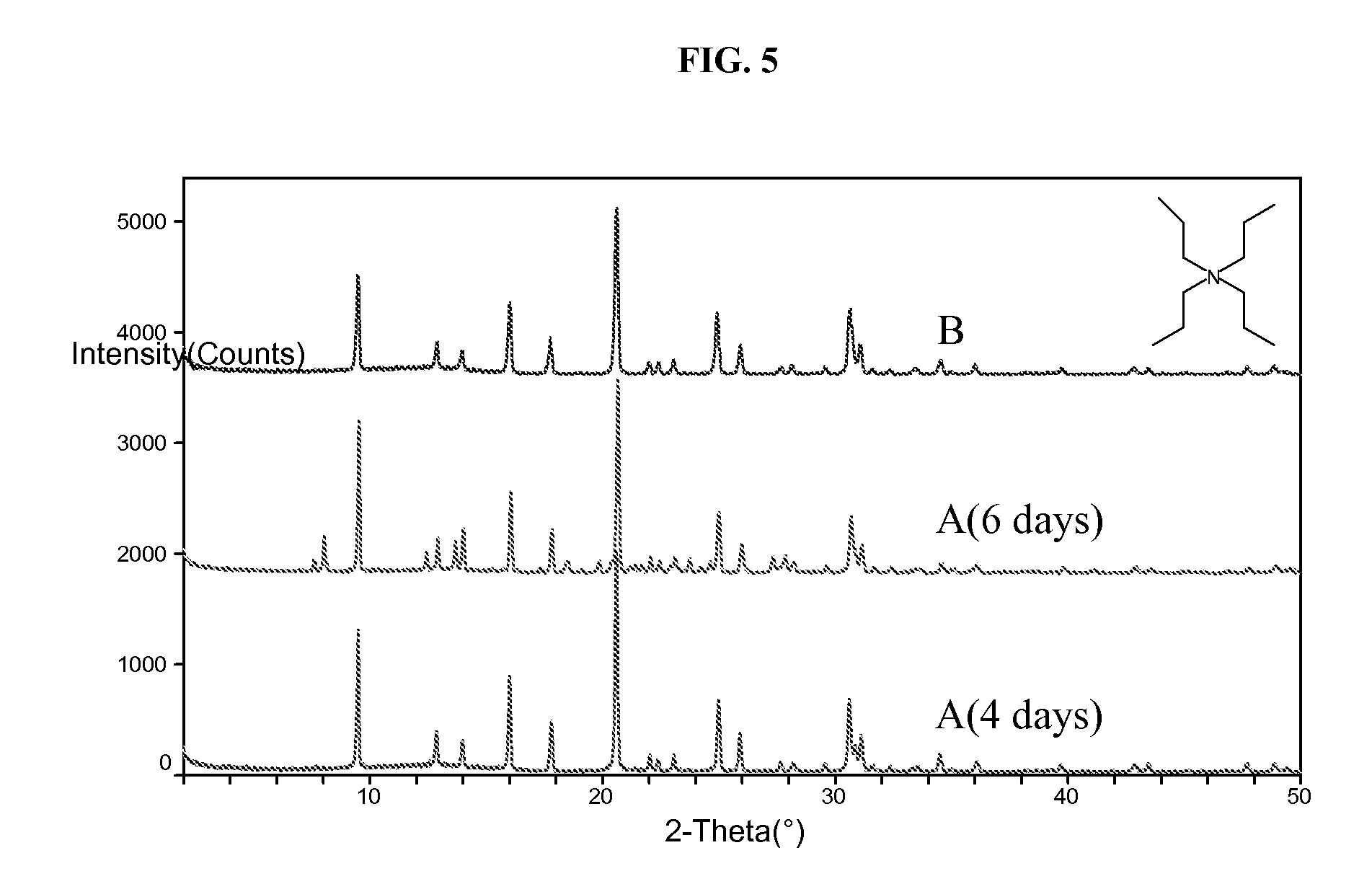
The synthesis of a crystalline material, in particular a high silica zeolite, having a chabazite-type framework is aided by the addition to the synthesis mixture of seeds of an AEI framework-type material. The chabazite-type product has a relatively small crystal size and exhibits activity and selectivity in the conversion of methanol to lower olefins, especially ethylene and propylene.
Owner:EXXONMOBIL CHEM PAT INC +1
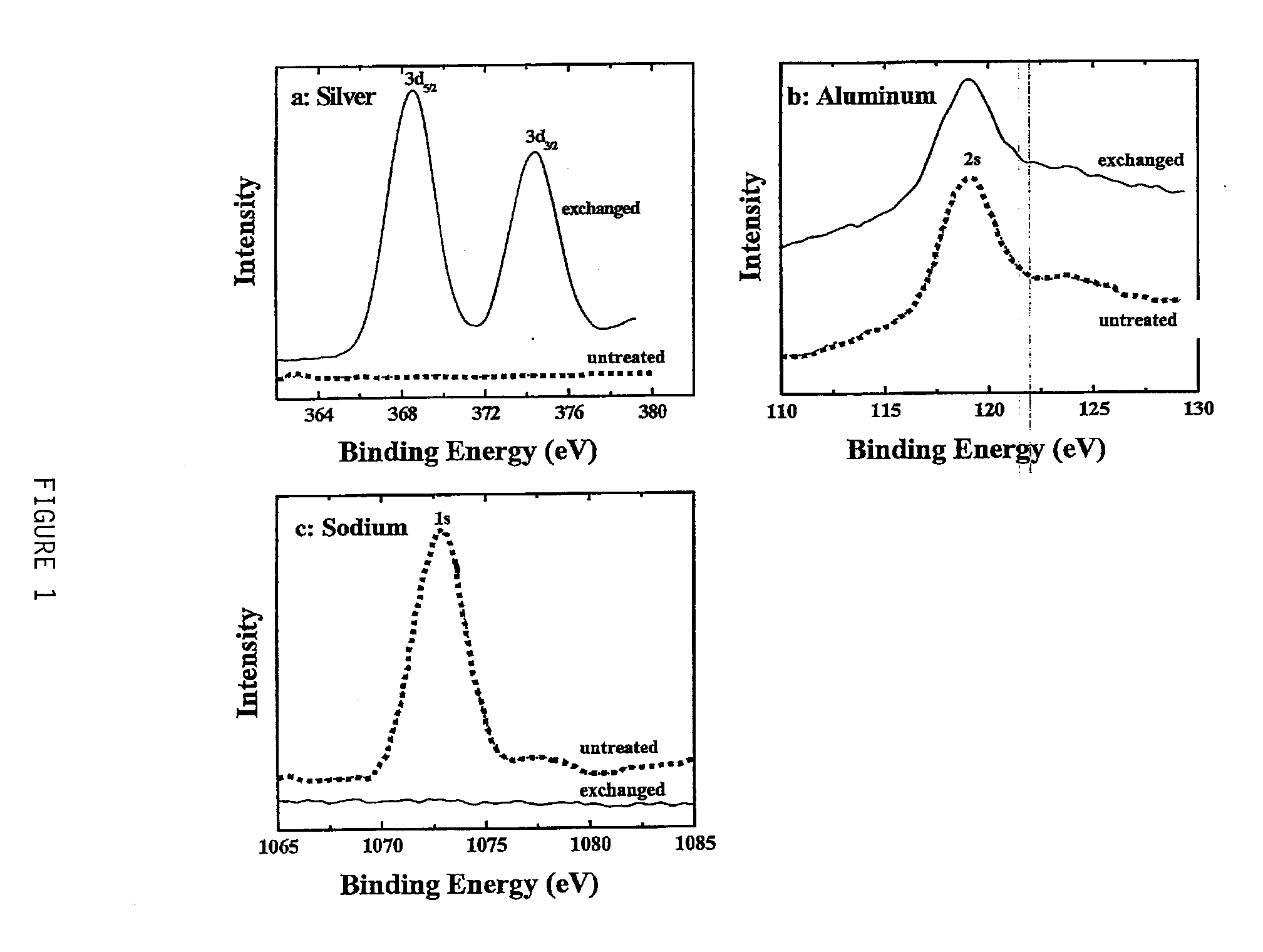
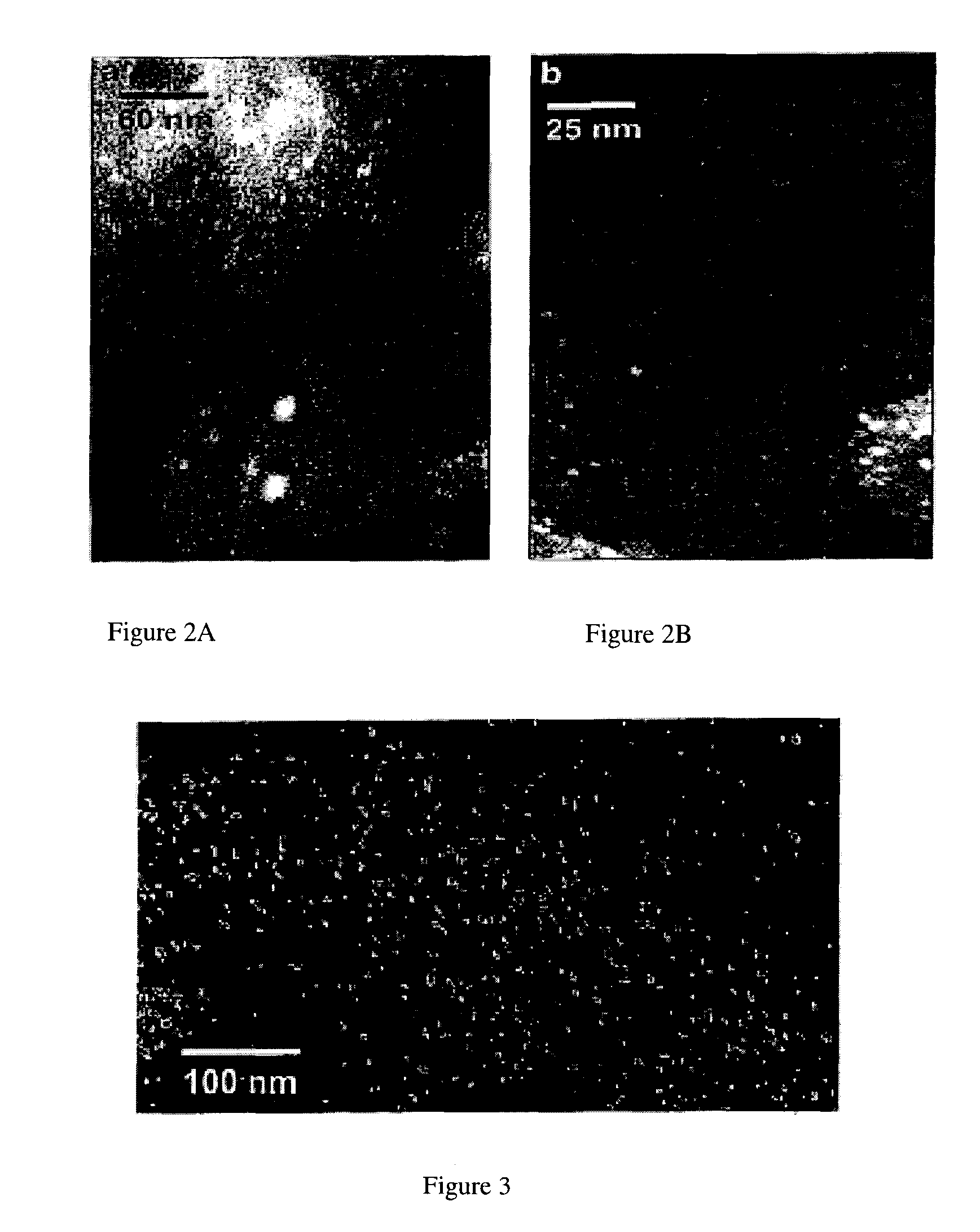
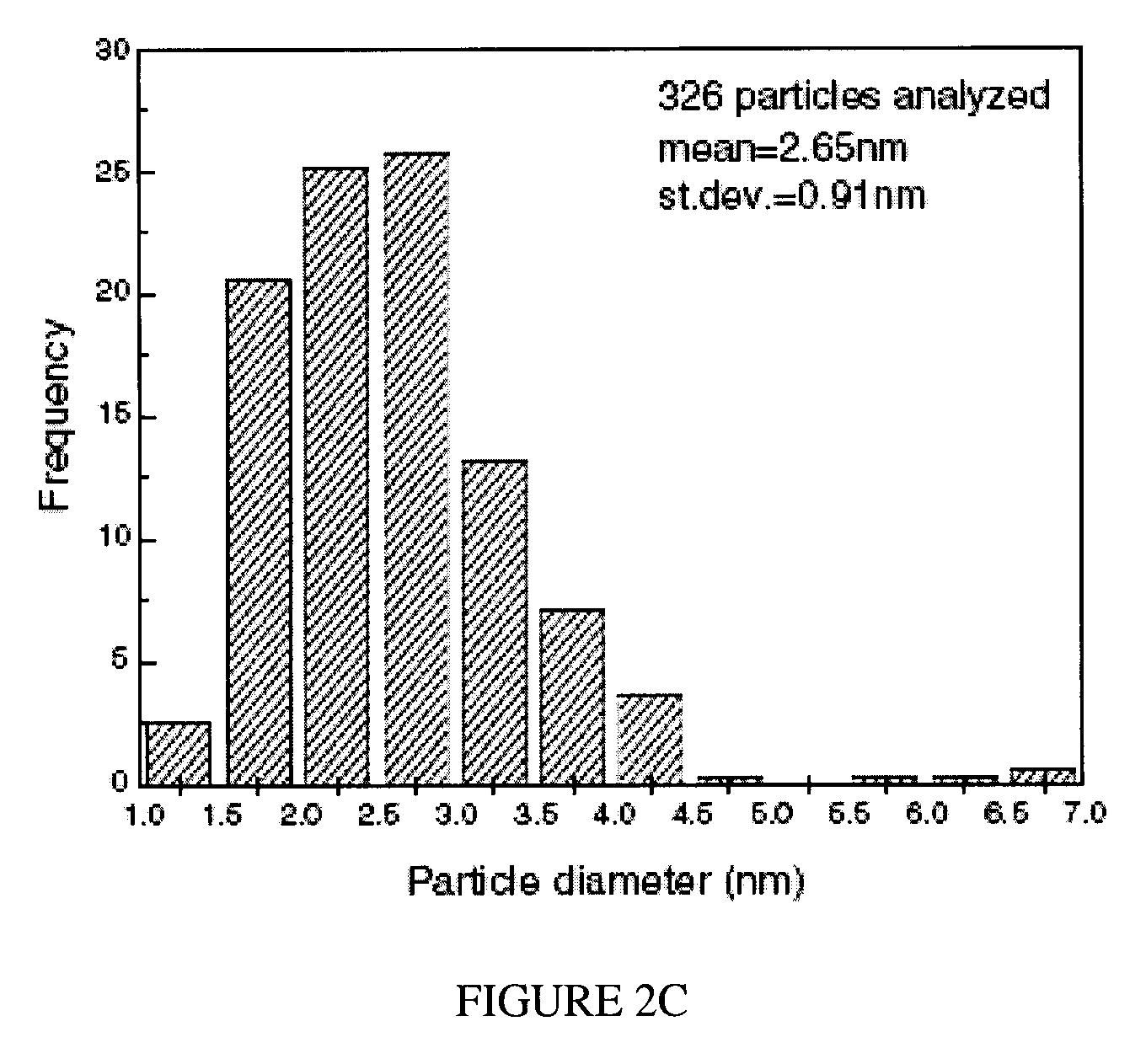
Mercury absorption using chabazite supported metallic nanodots
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
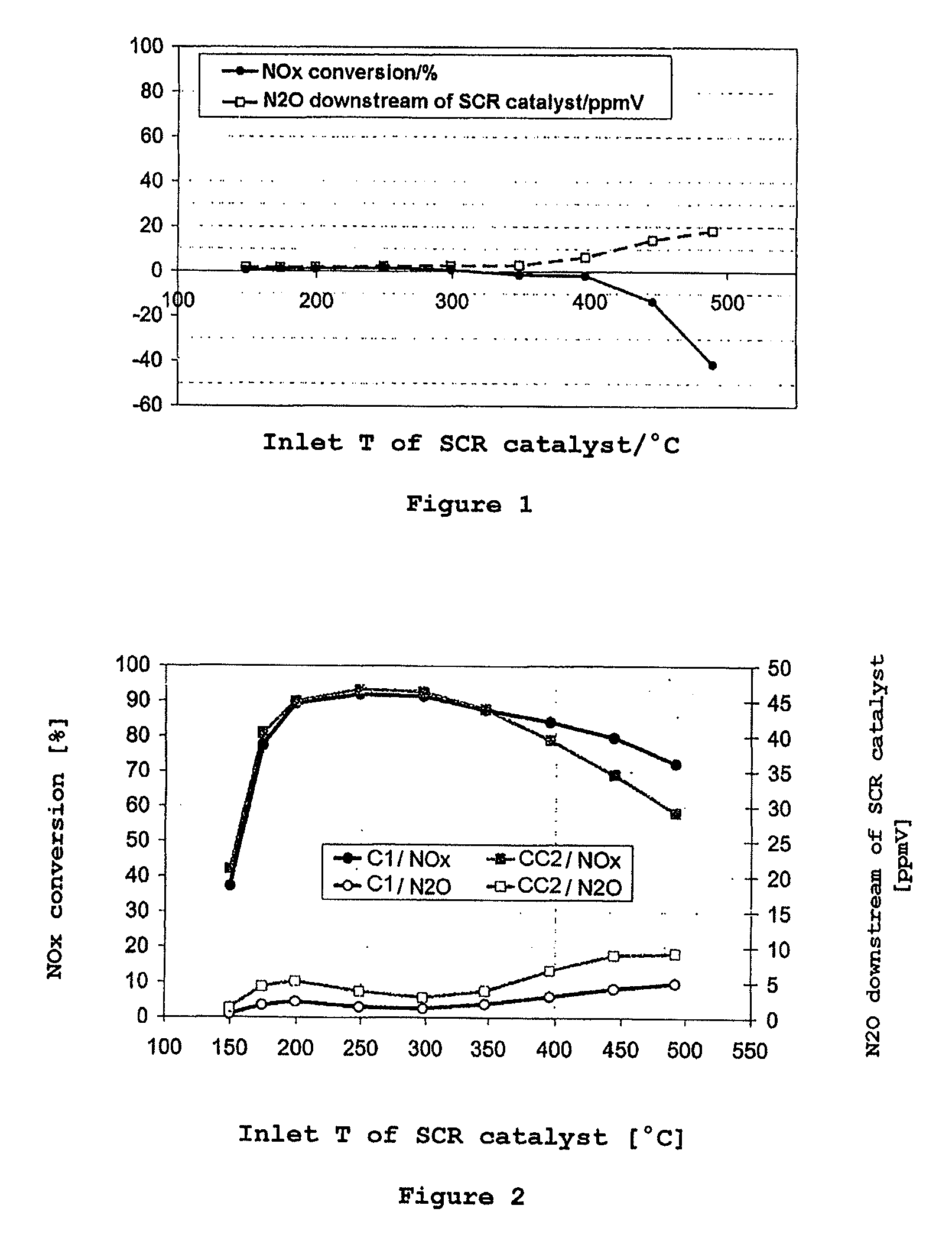
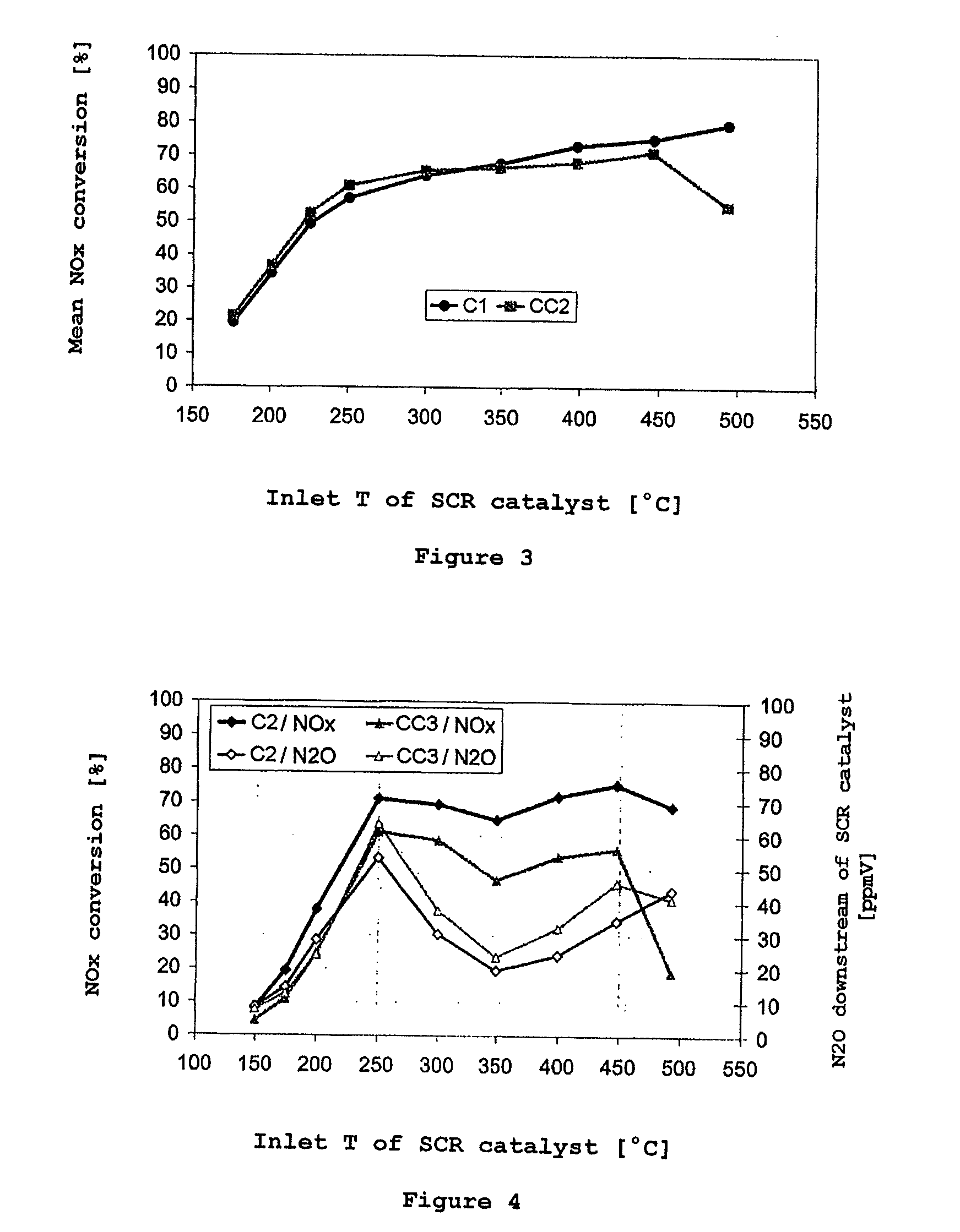
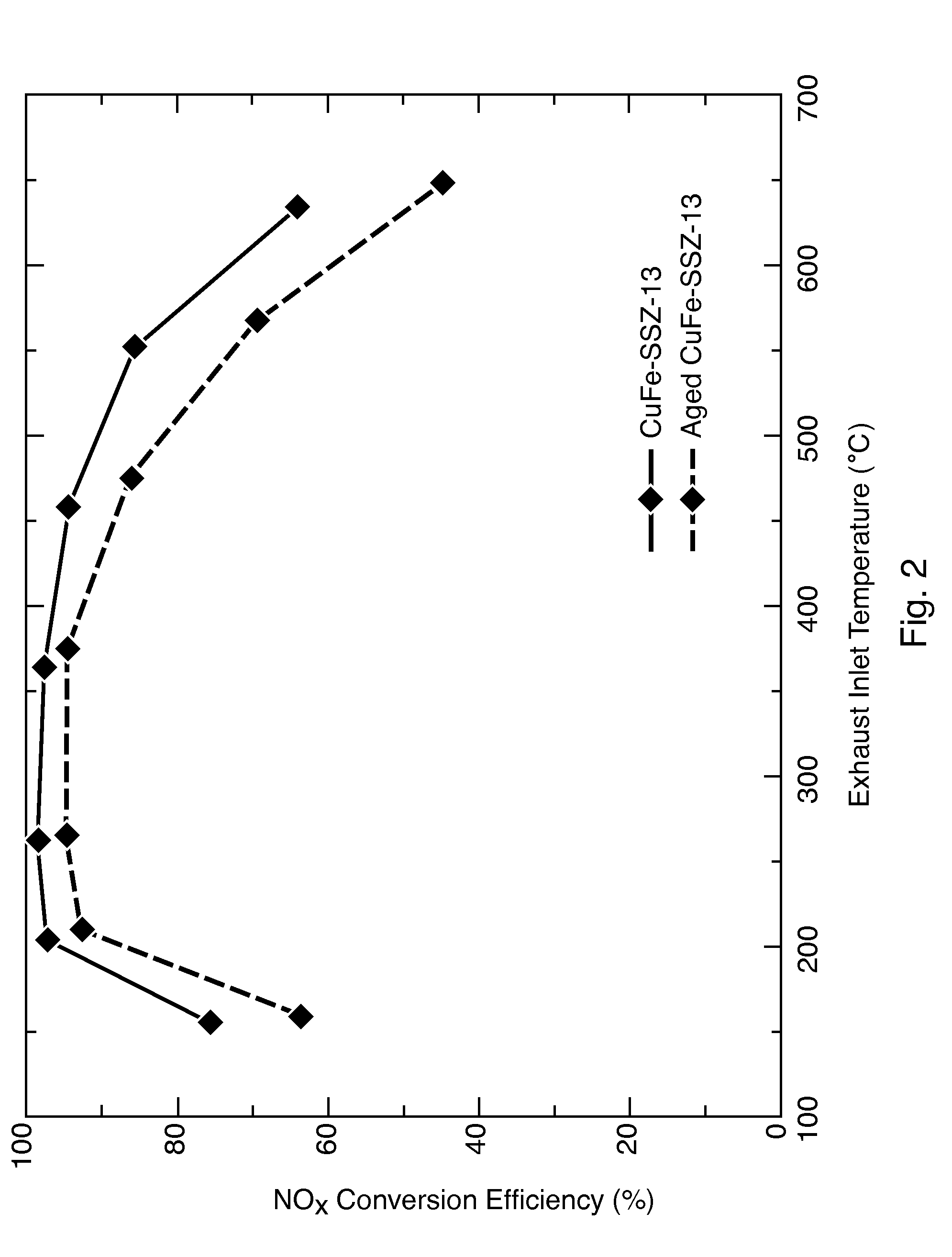
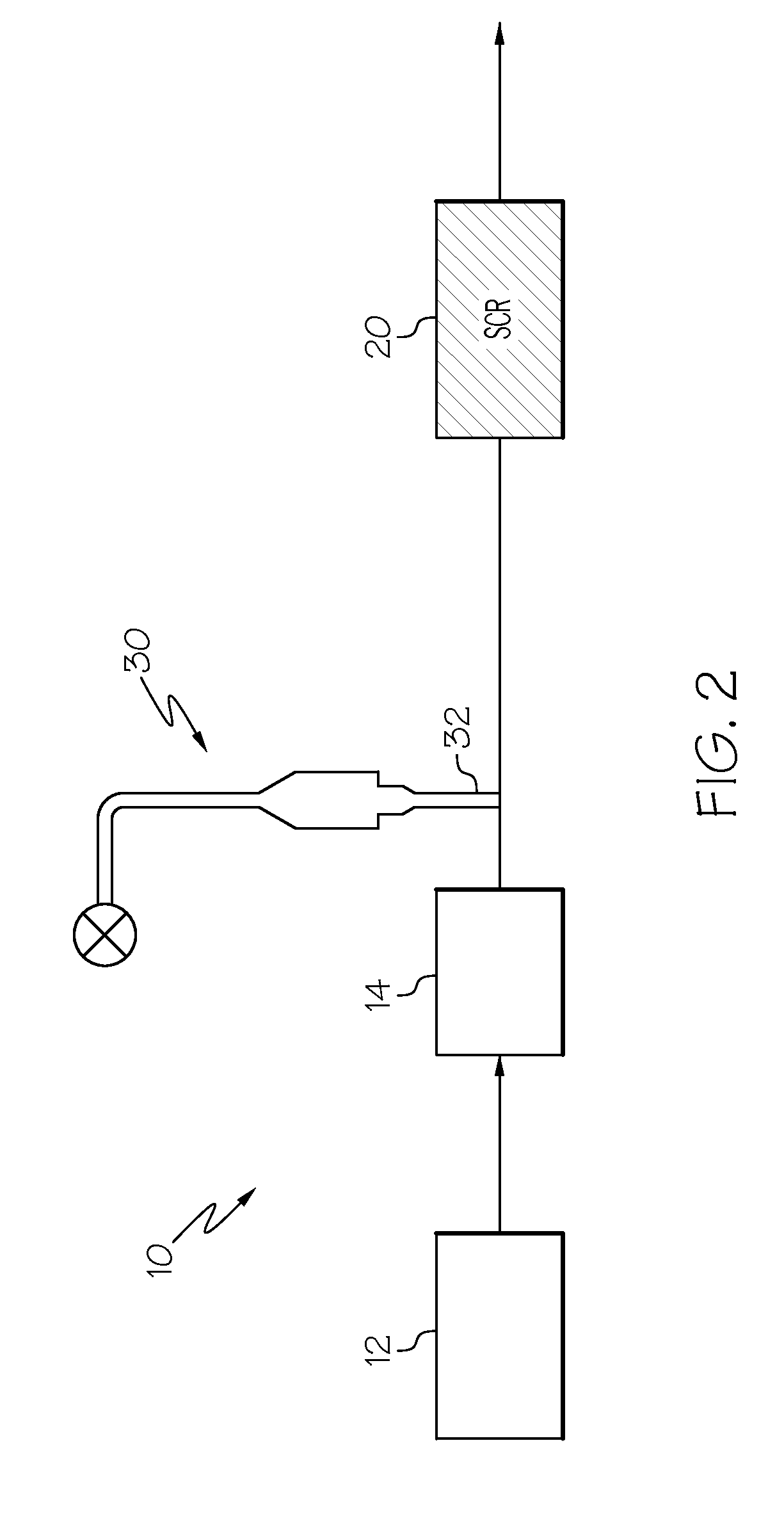
Selective catalytic reduction of nitrogen oxides in the exhaust gas of diesel engines
InactiveUS20110142737A1High activityGood choiceNitrogen compoundsInternal combustion piston enginesCeriumDiesel engine
A catalyst and a process for selective catalytic reduction of nitrogen oxides in diesel engine exhaust gases with ammonia or a compound decomposable to ammonia are described. The exhaust gas to be cleaned is passed together with ammonia or a compound decomposable to ammonia over a catalyst which comprises a zeolite or a zeolite-like compound containing 1-10% by weight of copper, based on the total weight of the zeolite or of the zeolite-like compound, and a homogeneous cerium-zirconium mixed oxide and / or a cerium oxide. The zeolite used or the zeolite-like compound used is selected from the group consisting of chabazite, SAPO-34, ALPO-34 and zeolite-β.
Owner:UMICORE AG & CO KG
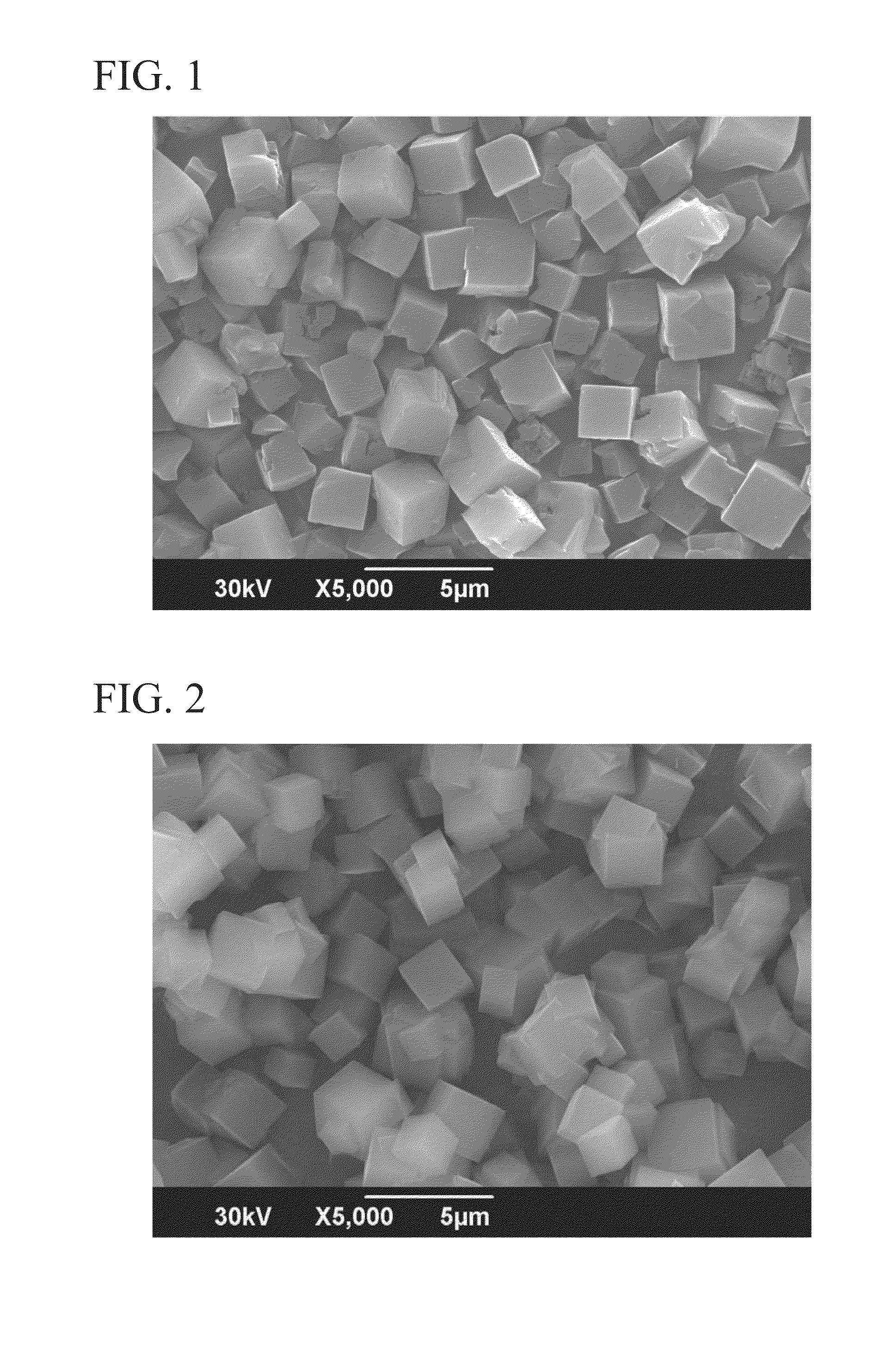
Large crystal, organic-free chabazite, methods of making and using the same
InactiveUS20120269719A1SAR of the product may be increasedAluminium compoundsGas treatmentOrganic structureCrystalline materials
Owner:PQ CORP (US)
Chabazite-type molecular sieve, its synthesis and its use in the conversion of oxygenates to olefins
The synthesis of a crystalline material, in particular a high silica zeolite, having a chabazite-type framework is aided by the addition to the synthesis mixture of seeds of an AEI framework-type material. The chabazite-type product has a relatively small crystal size and exhibits activity and selectivity in the conversion of methanol to lower olefins, especially ethylene and propylene.
Owner:EXXONMOBIL CHEM PAT INC +1
Chabazite-containing molecular sieve, its synthesis and its use in the conversion of oxygenates to olefins
A crystalline material substantially free of framework phosphorus and comprising a CHA framework type molecular sieve with stacking faults or at least one intergrown phase of a CHA framework type molecular sieve and an AEI framework type molecular sieve, wherein said material, in its calcined, anhydrous form, has a composition involving the molar relationship: (n)X2O3:YO2, wherein X is a trivalent element; Y is a tetravalent element; and n is from 0 to about 0.5. The material exhibits activity and selectivity in the conversion of methanol to lower olefins, especially ethylene and propylene.
Owner:EXXONMOBIL CHEM PAT INC +1
Method of cementing a well using composition containing zeolite
ActiveUS7137448B2Good physical propertiesImprove flexural strengthDrilling compositionSealing/packingNatrolitePortland cement
A cementitious composition for cementing an oil or gas well and which exhibits, when cured, increased flexural strength and a flexural strength to compressive strength ratio between from about 0.29 to about 0.80, contains a hydraulically-active cementitious material, such as Portland cement, and substantially spherical zeolite. Representative zeolites include natrolite, heulandite, analcime, chabazite, stilbite, and clinoptilolite. The weight percent of zeolite in the cement composition is generally less than or equal to 15 percent. In practice, a well bore may be cemented by pumping the activated slurry and pumping it within the well bore to a pre-selected location and allowing it to solidify.
Owner:BAKER HUGHES INC
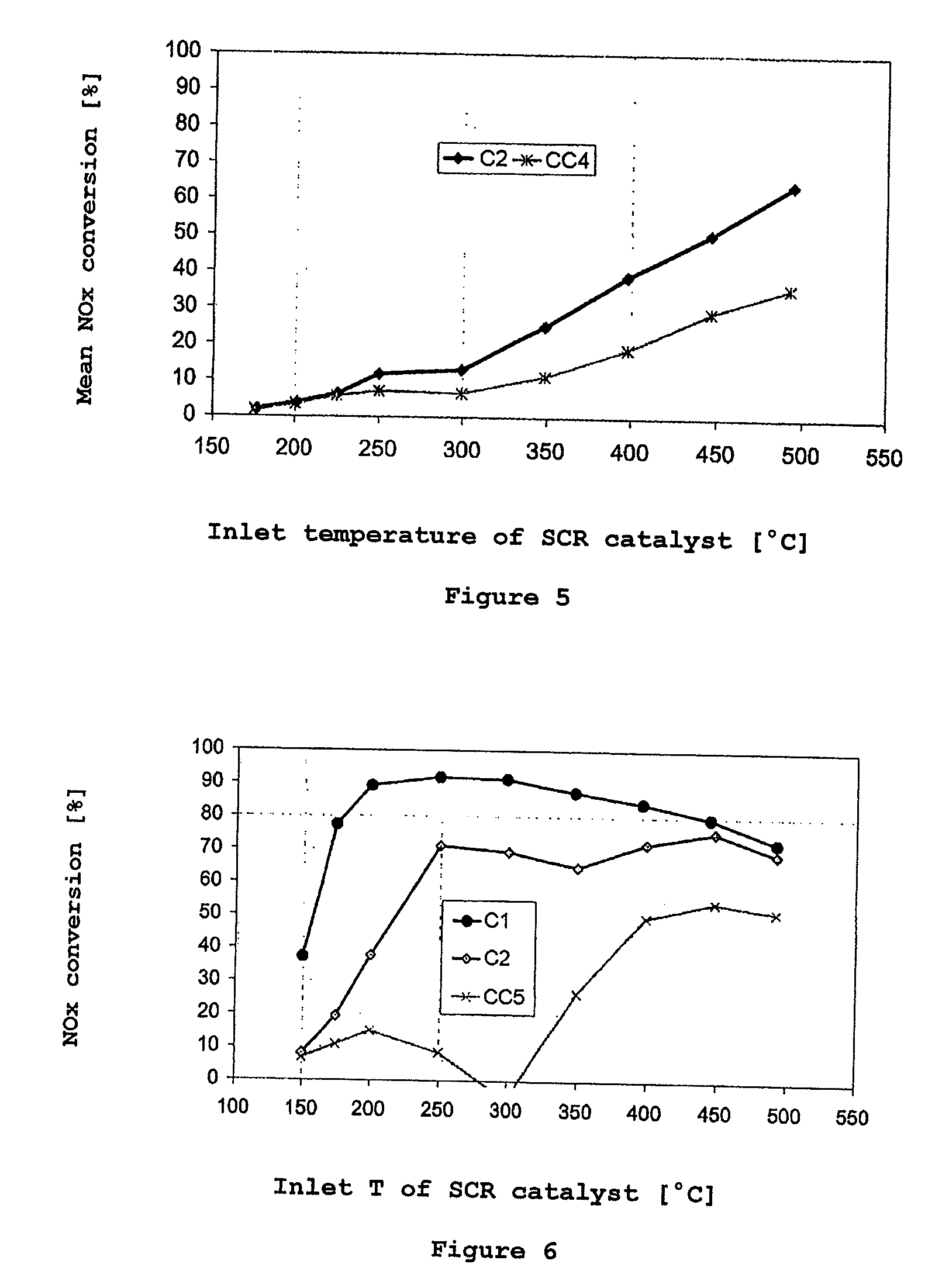
Chabazite-type zeolite and method for producing same, copper loaded low-silica zeolite and NOX reductive removal catalyst containing the zeolite, and method of NOX reductive removal using this catalyst
ActiveUS20130280160A1High levelIncrease resistanceAluminium compoundsNitrogen compoundsReduction rateHeat resistance
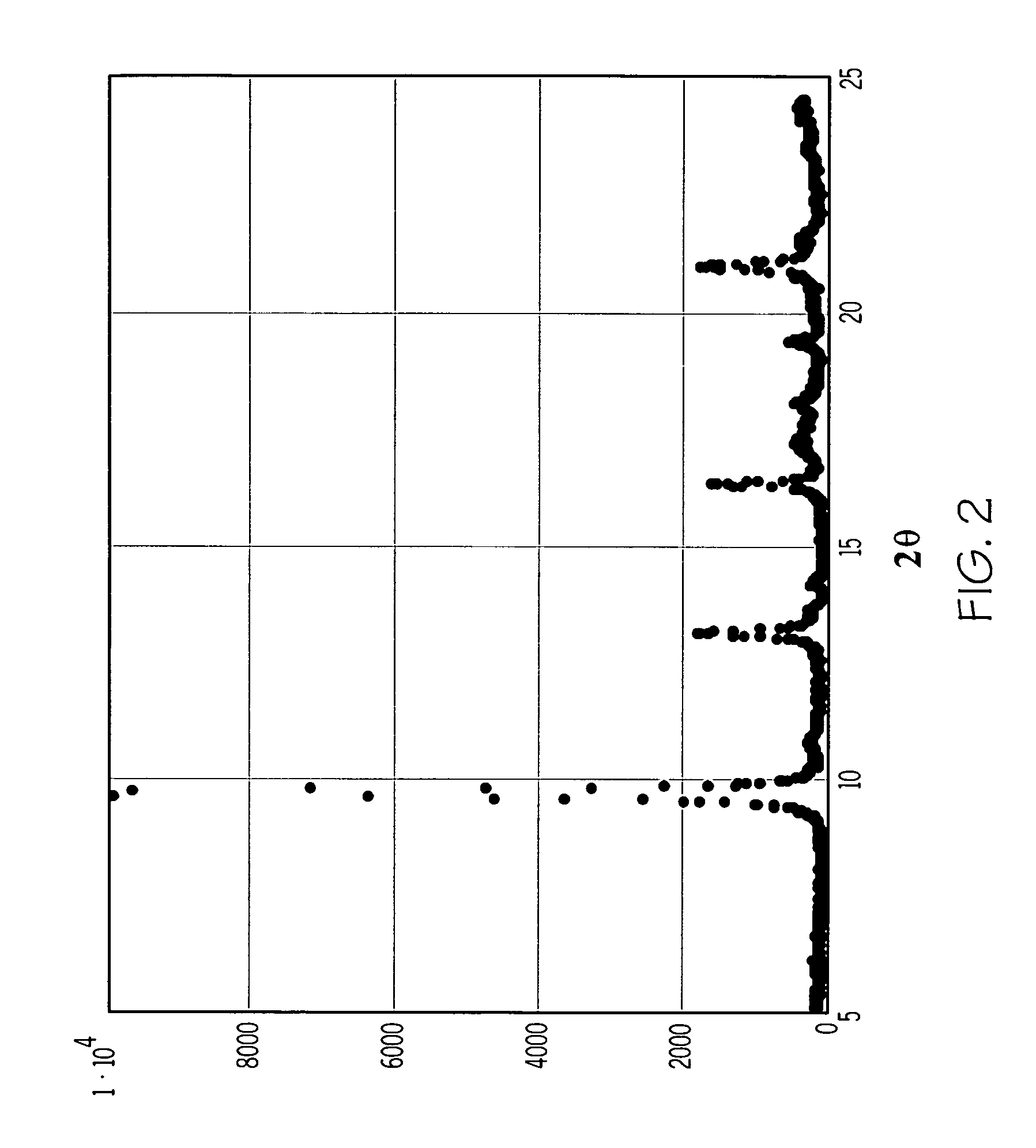
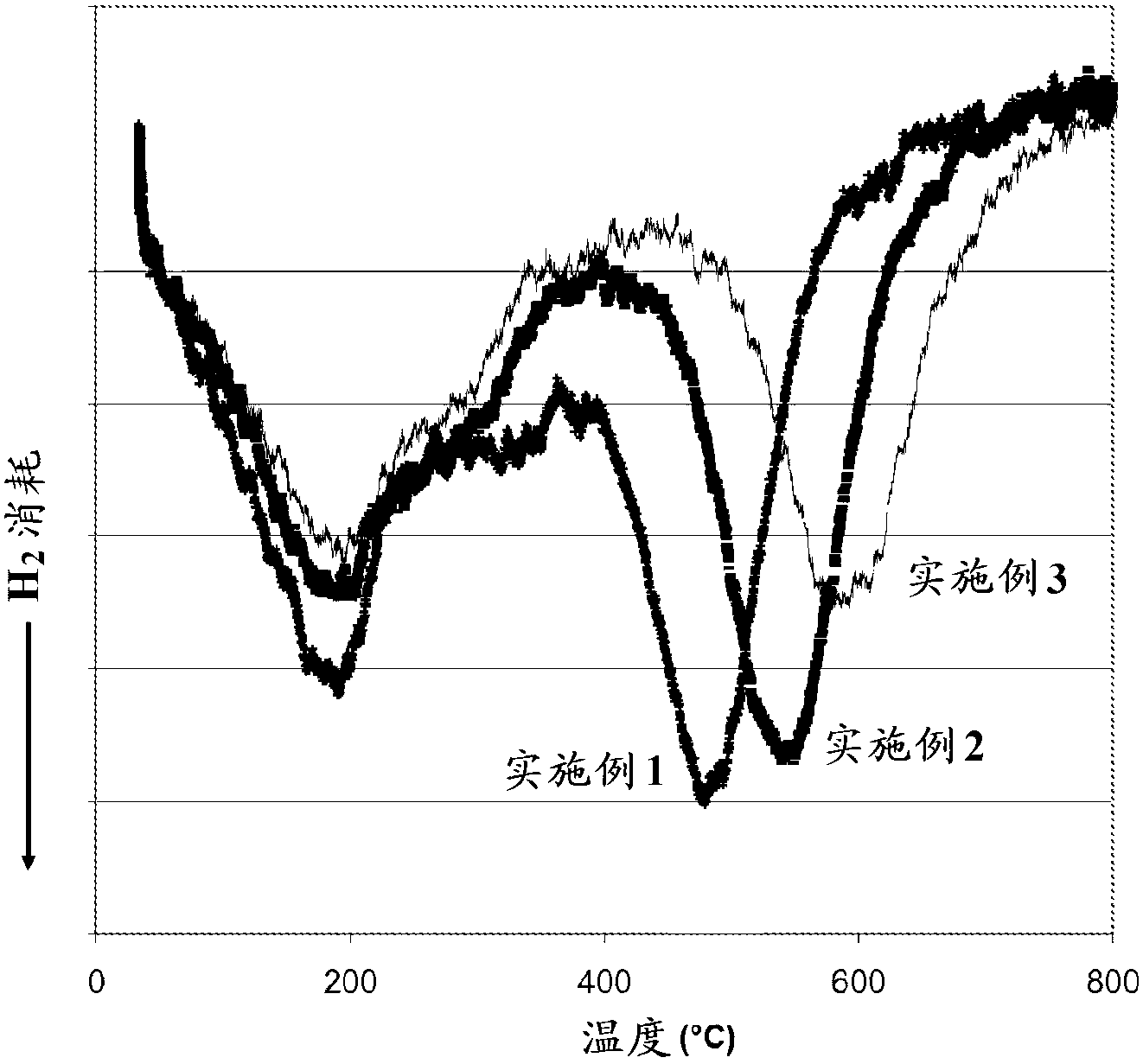
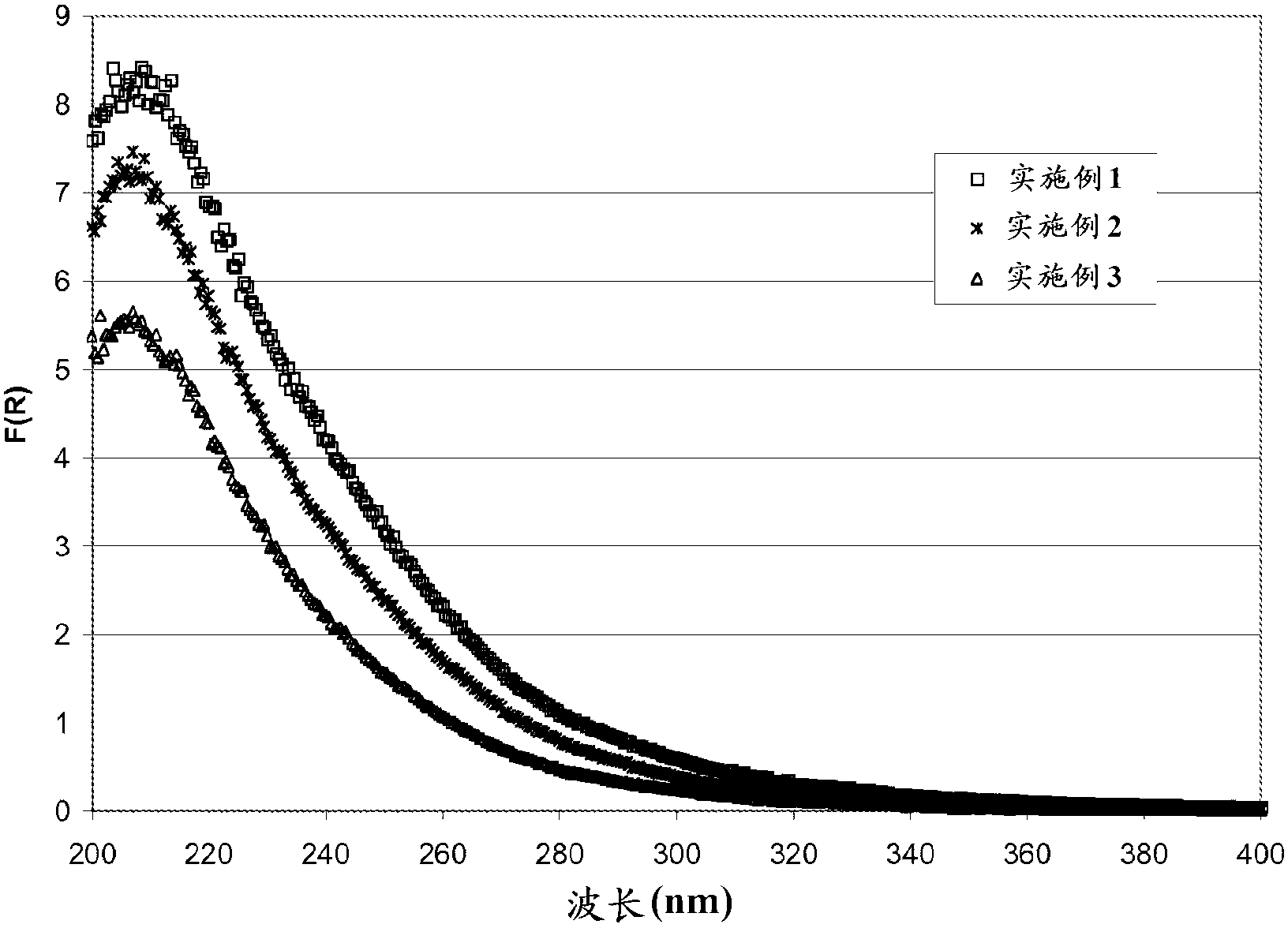
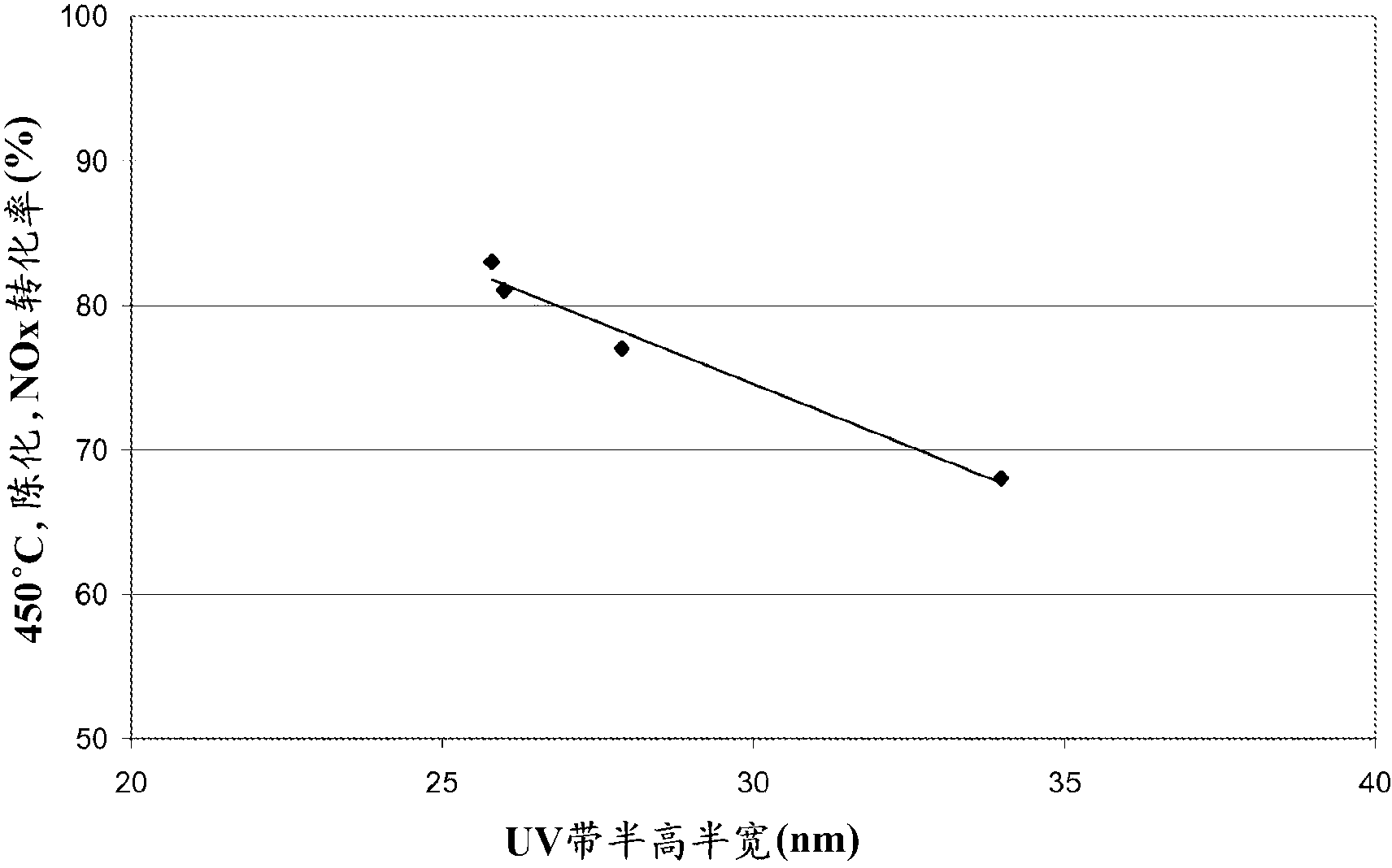
The chabazite-type zeolite of the present invention has a SiO2 / Al2O3 molar ratio of less than 15, and an average particle size from 1.0 μm to 8.0 μm. The chabazite-type zeolite of the present invention has excellent durability and heat resistance, and the copper-loaded chabazite-type zeolite has an improved NOx reduction rate at low temperatures compared to conventional copper-loaded chabazite-type zeolite.
Owner:TOSOH CORP
Method for synthesizing chabazite-type molecular sieve with high silica-alumina ratio by using seed crystal and composite inorganic base and application thereof
ActiveCN104129800AReduce water usageHigh solid contentMolecular sieve catalystsHydrocarbon from oxygen organic compoundsMolecular sievePotassium hydroxide
A method synthesizing a chabazite-type molecular sieve with high silica-alumina ratio by using seed crystal and composite inorganic base is as below: evenly mixing an alkaline silica sol and a template; slowly adding potassium hydroxide; adding sodium hydroxide; continuing to stir; then slowly adding an aqueous solution of aluminum sulfate; adding a chabazite crystal phase molecular sieve as the seed crystal; distilling to remove excessive water; sealing, insulating and stirring; conducting high speed shearing to obtain a uniform gel; and conducting crystallization, roasting and ammonium exchange to obtain a product. The invention has the advantages of high silica-alumina ratio, high specific surface area, high MTO diolefin selectivity and pollution-free preparation process.
Owner:天津众智科技有限公司
Synthesis of chabazite-containing molecular sieves and their use in the conversion of oxygenates to olefins
The synthesis of a crystalline material, in particular, a high silica zeolite, comprising a chabazite-type framework molecular sieve is conducted in the presence of an organic directing agent having the formula:[R1R2R3N—R4]+Q−wherein R1 and R2 are independently selected from hydrocarbyl groups and hydroxy-substituted hydrocarbyl groups having from 1 to 3 carbon atoms, provided that R1 and R2 may be joined to form a nitrogen-containing heterocyclic structure, R3 is an alkyl group having 2 to 4 carbon atoms and R4 is selected from a 4- to 8-membered cycloalkyl group, optionally, substituted by 1 to 3 alkyl groups each having from 1 to 3 carbon atoms; and a 4- to 8-membered heterocyclic group having from 1 to 3 heteroatoms, said heterocyclic group being, optionally, substituted by 1 to 3 alkyl groups each having from 1 to 3 carbon atoms and the or each heteroatom in said heterocyclic group being selected from the group consisting of O, N, and S, or R3 and R4 are hydrocarbyl groups having from 1 to 3 carbon atoms joined to form a nitrogen-containing heterocyclic structure; and Q− is a anion.
Owner:EXXONMOBIL CHEM PAT INC
Method of Controlling Organoleptic Odors
InactiveUS20100189595A1Reduce odorMolecular sieve catalystsDeodrantsCalcium silicateSodium Bentonite
A method is taught for capturing organoleptic odor. Where a functional additive has odors from a plurality of organolepic sources, and is blended with an odor control agent and a resin to produce a blend, where the blend exhibits at least a 5% reduction in odor based on a standardized odor test SAE-J1351. The odor control agent is selected from the group but not limited to: nepheline syenite, silica gel, hydrogels, hard and soft clays, bentonite, clinoptilolite, hectorite, cationic exchanged clinoptilolites, cerium, cesium, chabazite, faujasite, gmelinite, brewsterite, calcium silicate, hydrotalcites, zinc or magnesium aluminum hydroxy carbonates, zinc oxide, zinc hydroxide, zinc carbonate, calcium oxide, calcium hydroxide, calcium carbonate, potassium meta phosphate, silver oxide, magnesium hydroxide, magnesium oxide, copper oxide, ferric and ferrous oxides, sorbitol, glucitol, mannitol, glucose, dextrose, dextrin, allophanes, silica, sodalite, silicon oxide, aluminum oxide, natural zeolites, manganese dioxide, nano zinc oxide and nano titanium and combination thereof.
Owner:LEHIGH TECH INC
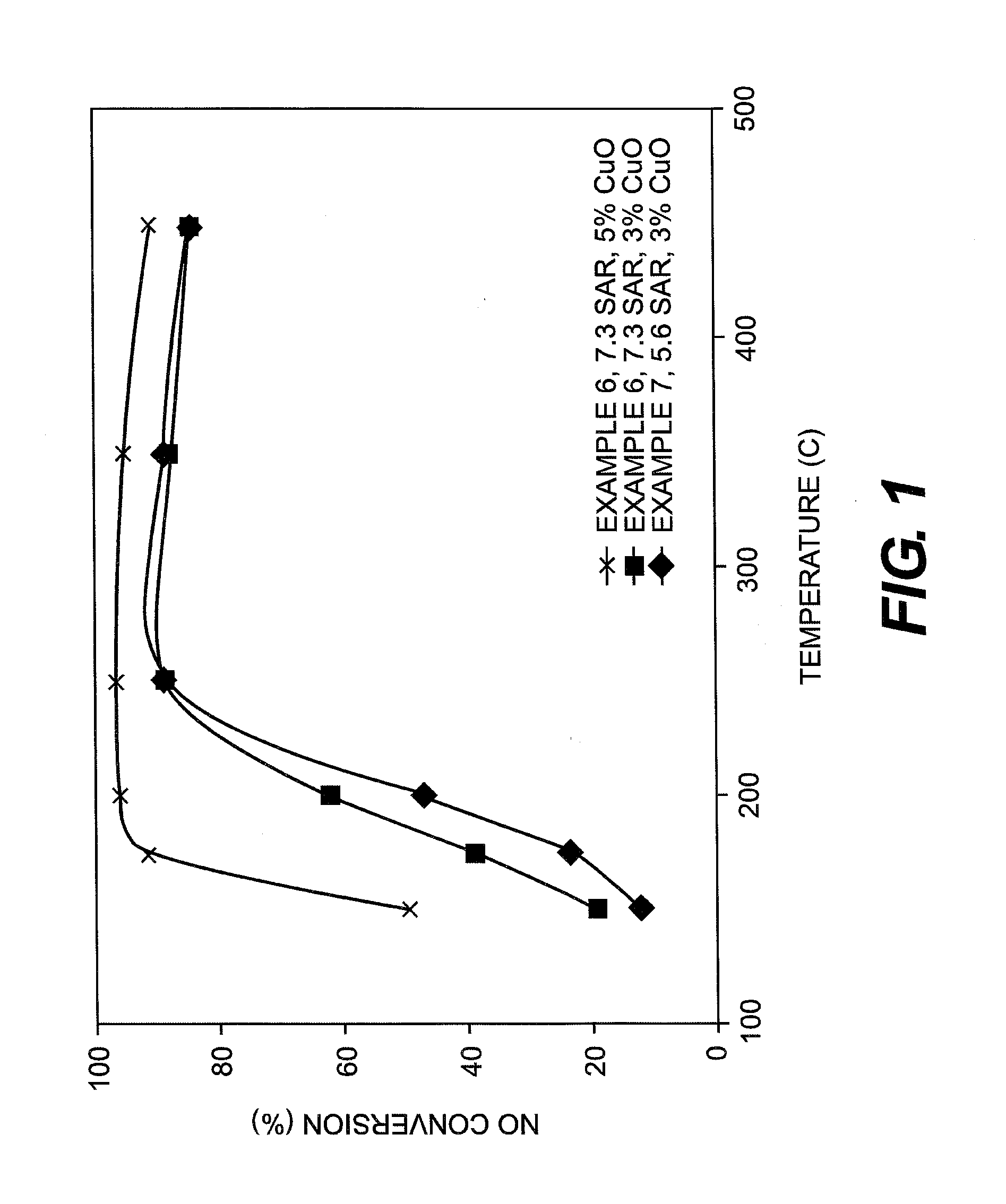
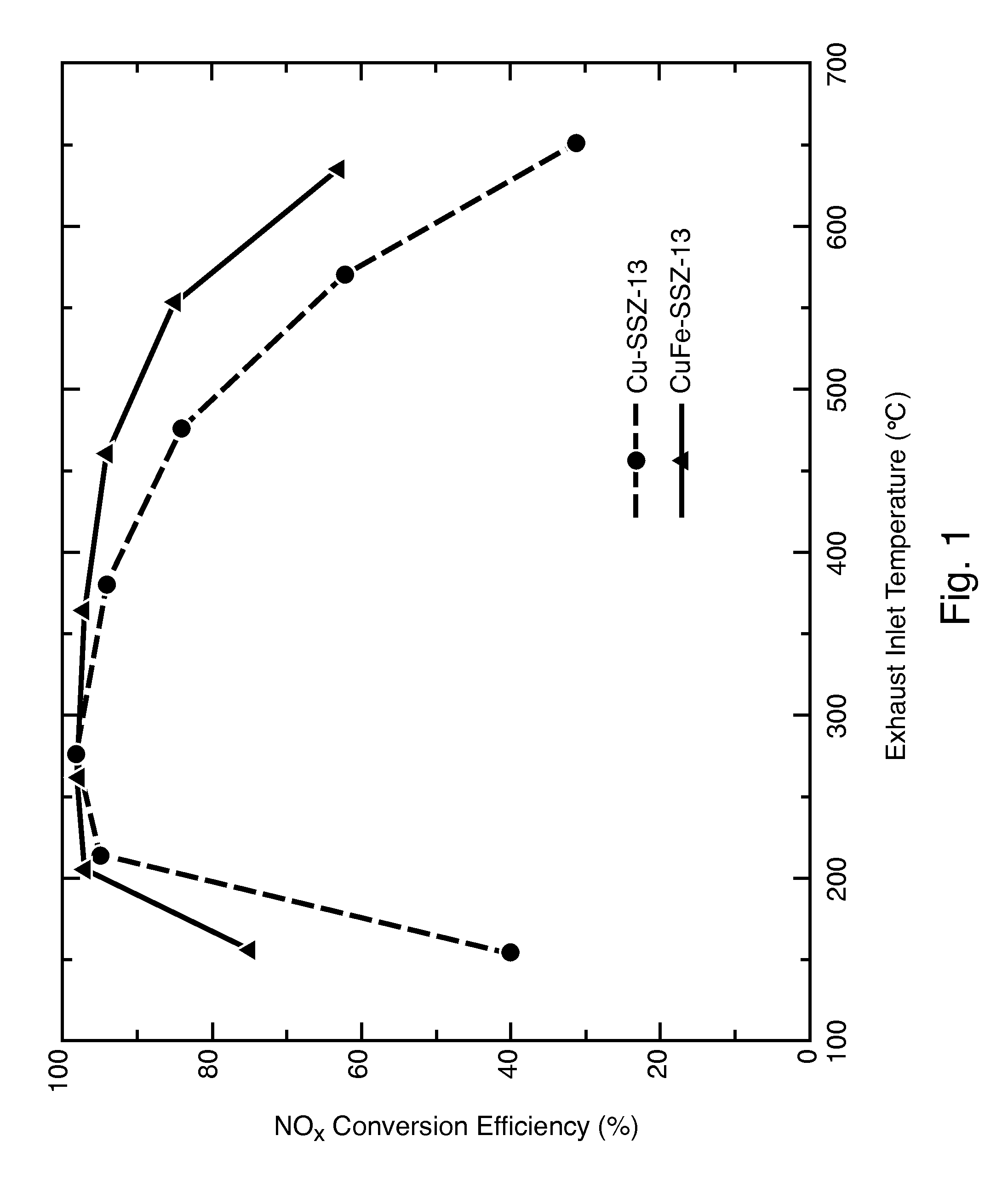
Hydrothermally Stable, Low-Temperature NOx Reduction NH3-SCR Catalyst
ActiveUS20130224082A1Reduce nitrogen oxide emissionsSuitable for operationCombination devicesGas treatmentPhysical chemistryExhaust fumes
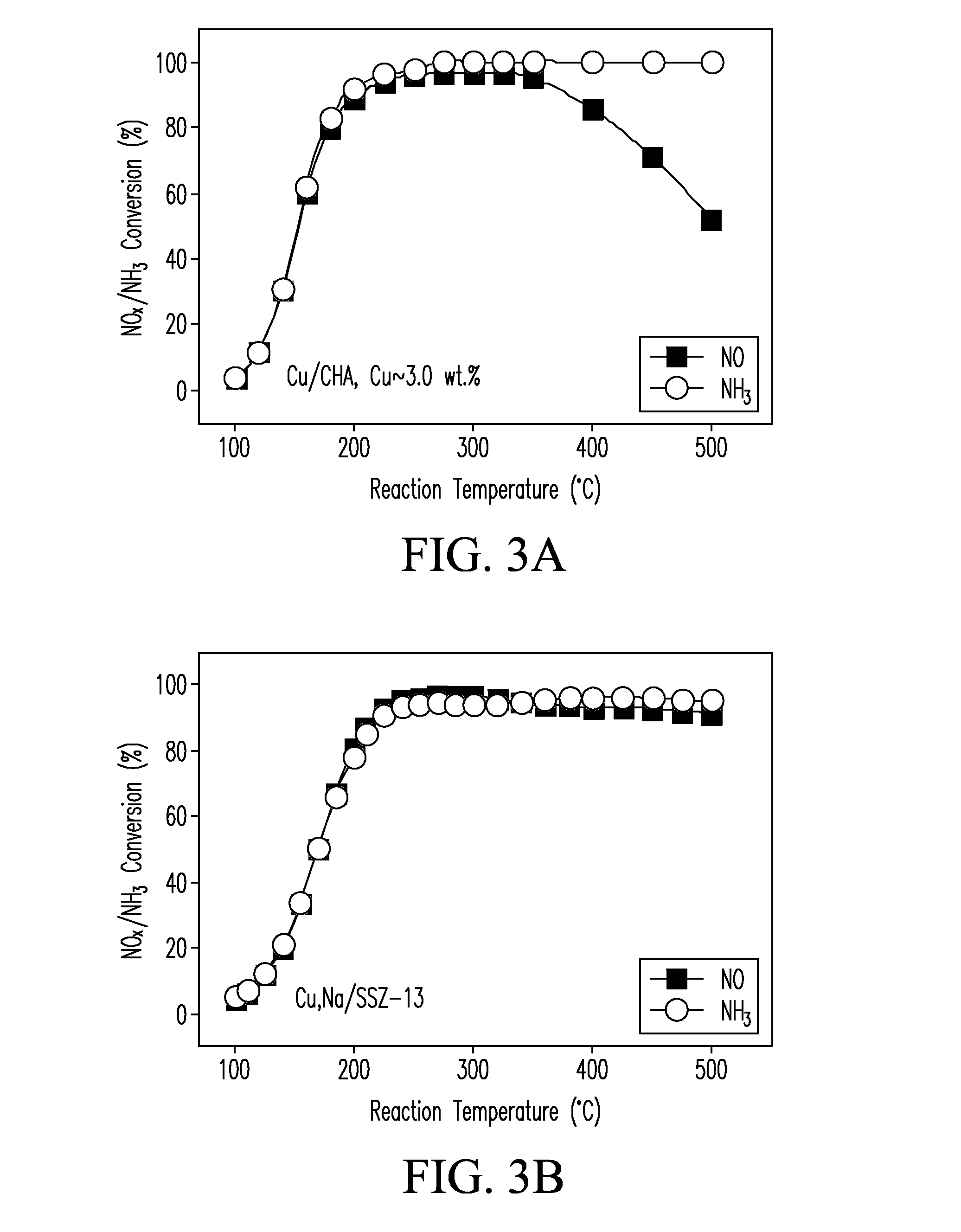
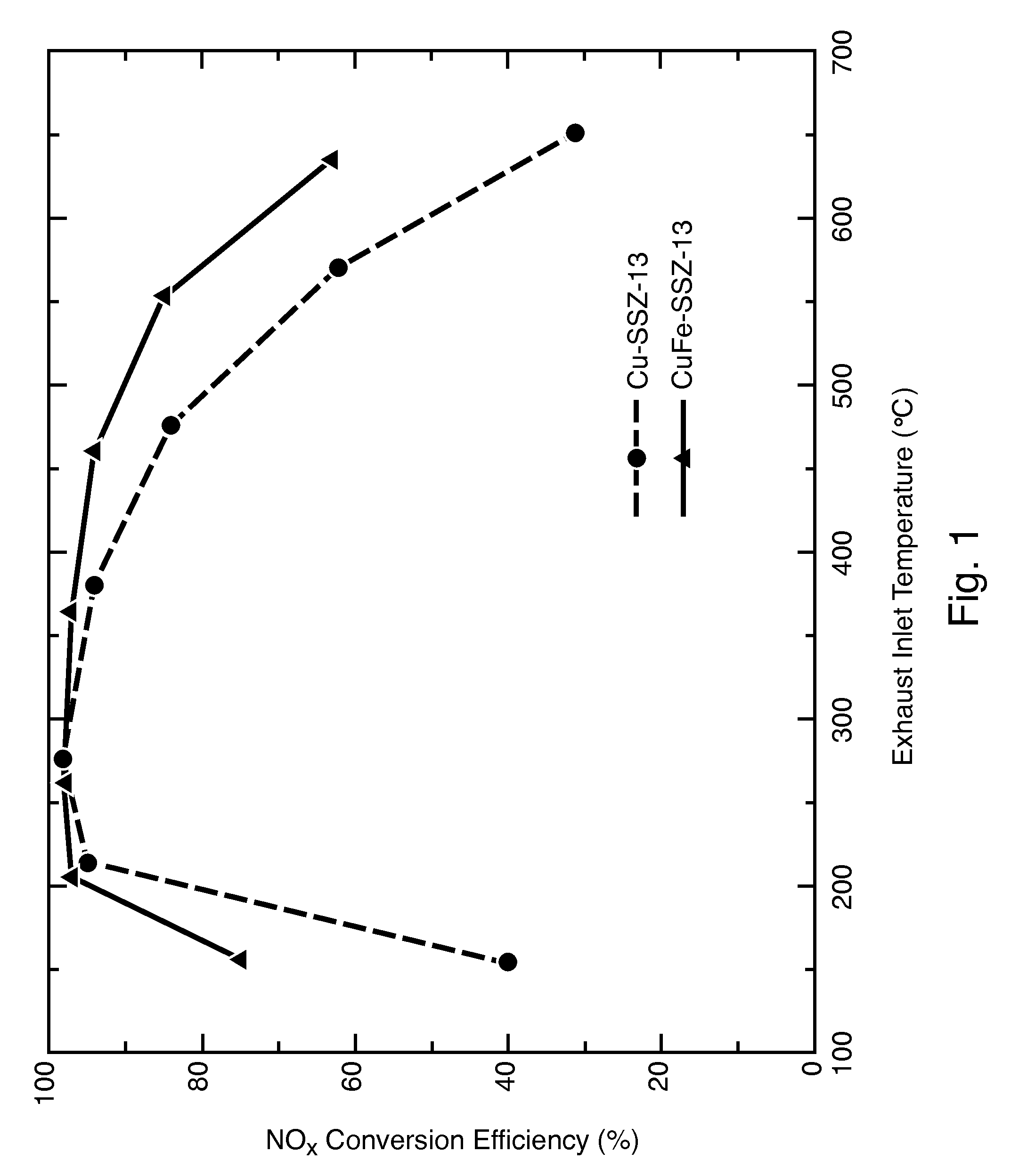
A catalyst composition includes a heterobimetallic zeolite characterized by a chabazite structure loaded with copper ions and at least one trivalent metal ion other than Al3+. The catalyst composition decreases NOx emissions in diesel exhaust and is suitable for operation in a catalytic converter.
Owner:UT BATTELLE LLC
Synthesis Of Chabazite-Containing Molecular Sieves And Their Use In The Conversion Of Oxygenates To Olefins
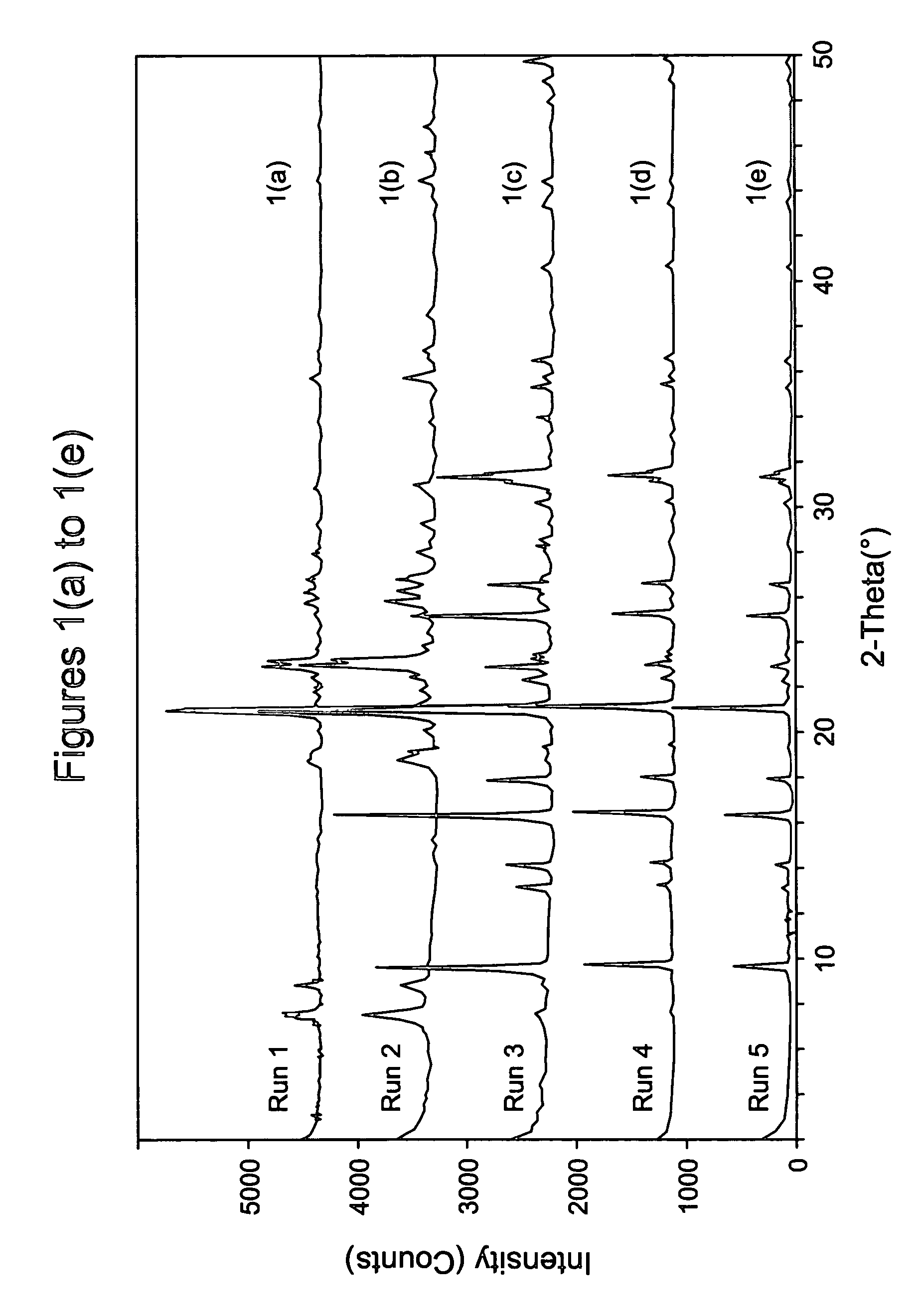
In a method of synthesizing a mostly CHA-type silicoaluminophosphate sieve, a reaction mixture comprises sources of water, silicon, aluminum, phosphorus, and a template. In one aspect, the inorganic phosphorus and silicon sources are first combined to form a primary mixture that is aged. Then, the aluminum source is added, followed optionally by any organic phosphorus source, and then the template, to form the synthesis mixture. After heating at <10° C. / hr to induce crystallization, in this aspect, both the crystallized sieve has an average crystal size ≦1.5 μm and / or is recovered in a yield of ≧10.0 wt %. In another aspect, when the synthesis mixture Si / Al2 ratio is <0.33, crystallization is induced. Advantageously, the sieve so crystallized has a template efficiency of ≧0.5 and / or is recovered in a yield of ≧10.0 wt %. The molecular sieve from both aspects can be used in a hydrocarbon (oxygenates-to-olefins) conversion process.
Owner:EXXONMOBIL CHEM PAT INC
Crystalline silicoaluminophosphate salt molecular sieve having octaoxygen-membered ring pore, process for producing the same and process for producing methylamine with the molecular sieve as catalyst
InactiveUS20050249661A1High activity selectivityStable in dimethylamine selectivityAluminium compoundsPhosphatesPresent methodOxygen
Problems on catalyst production and catalyst performance with respect to conventional 8-oxygen-membered ring micropore-containing crystalline silicoaluminophosphate molecular sieves as non-equilibrium methylamine synthesis catalysts, are resolved. A chabazite type crystalline silicoaluminophosphate molecular sieve having high purity and high crystallinity and having, on a crystal grain surface, an amorphous oxide layer whose Si / Al atomic ratio is greater than that of the whole crystal grain can be stably produced with high yield with the use of a small amount of structure directing agents by the present method characterized in that hydrothermal treatment conducted in the production of 8-oxygen-membered ring micropore-containing crystalline silicoaluminophosphate sieves is controlled under specified treating conditions. The thickness and composition of the amorphous oxide layer, which exert marked influence on the yield of dimethylamine synthesis, can be easily controlled and reproduced under the conditions of catalyst synthesis according to the invention. Thus, the catalyst of high performance can be stably supplied by the present invention at a low cost with reduced output of waste.
Owner:MITSUBISHI GAS CHEM CO INC
IRON-ZEOLITE CHABAZITE CATALYST FOR USE IN NOx REDUCTION AND METHOD OF MAKING
InactiveUS20150231620A1Good high-temperature activityReduction of nitrogen oxidesCombination devicesAluminium compoundsNitrogen oxidesIon exchange
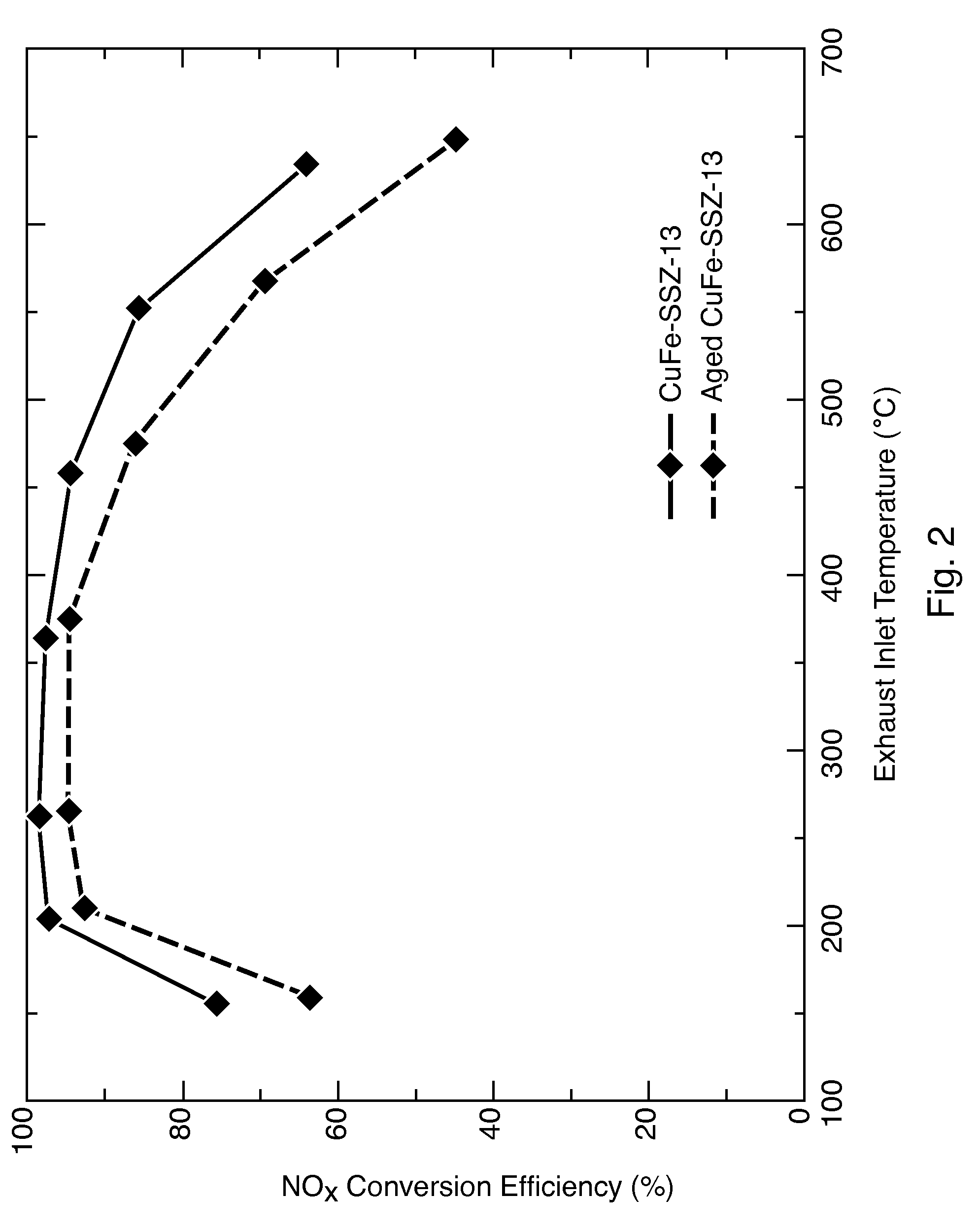
An iron-zeolite chabazite (CHA) catalyst is provided as an SCR catalyst for reducing nitrogen oxides (NOx) from vehicle engine exhausts. The catalyst is formed by incorporating iron during synthesis of the chabazite zeolite, which eliminates the need for a post-synthesis ion-exchange step and which results in the incorporation of iron into the (CHA) zeolite crystal lattice structure. The resulting catalyst exhibits good high temperature activity at temperatures greater than 550° C. and exhibits good thermal stability.
Owner:FORD GLOBAL TECH LLC
Process of direct copper exchange into na+-form of chabazite molecular sieve, and catalysts, systems and methods
InactiveCN102946997AShorten the timeHigh copper utilizationNitrous oxide captureGas treatmentMolecular sieveLiquid copper
Disclosed are processes for the preparation of copper containing molecular sieves with the CHA structure wherein the copper is exchanged into the Na+-form of the Chabazite, using a liquid copper solution wherein the concentration of copper is in the range of about 0.001 to about 0.4 molar. Also described are copper containing molecular sieves with the CHA structure, catalysts incorporating molecular sieves, systems and methods for their use.
Owner:BASF CORP
CATALYSTS FOR ENHANCED REDUCTION OF NOx GASES AND PROCESSES FOR MAKING AND USING SAME
InactiveUS20160107119A1High catalytic activityHigh selectivityNitrous oxide captureAluminium compoundsAlkali ionsHigh selectivity
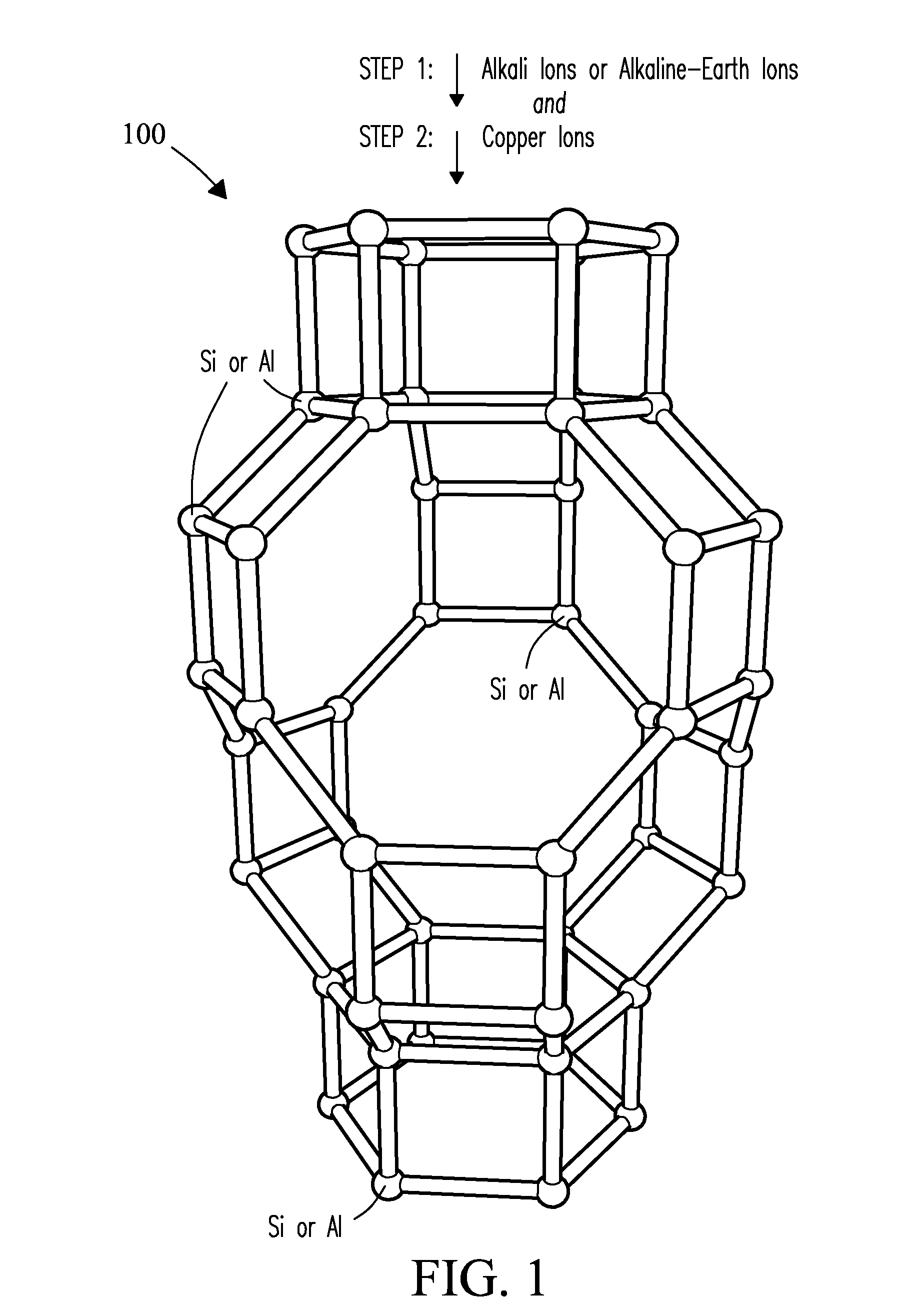
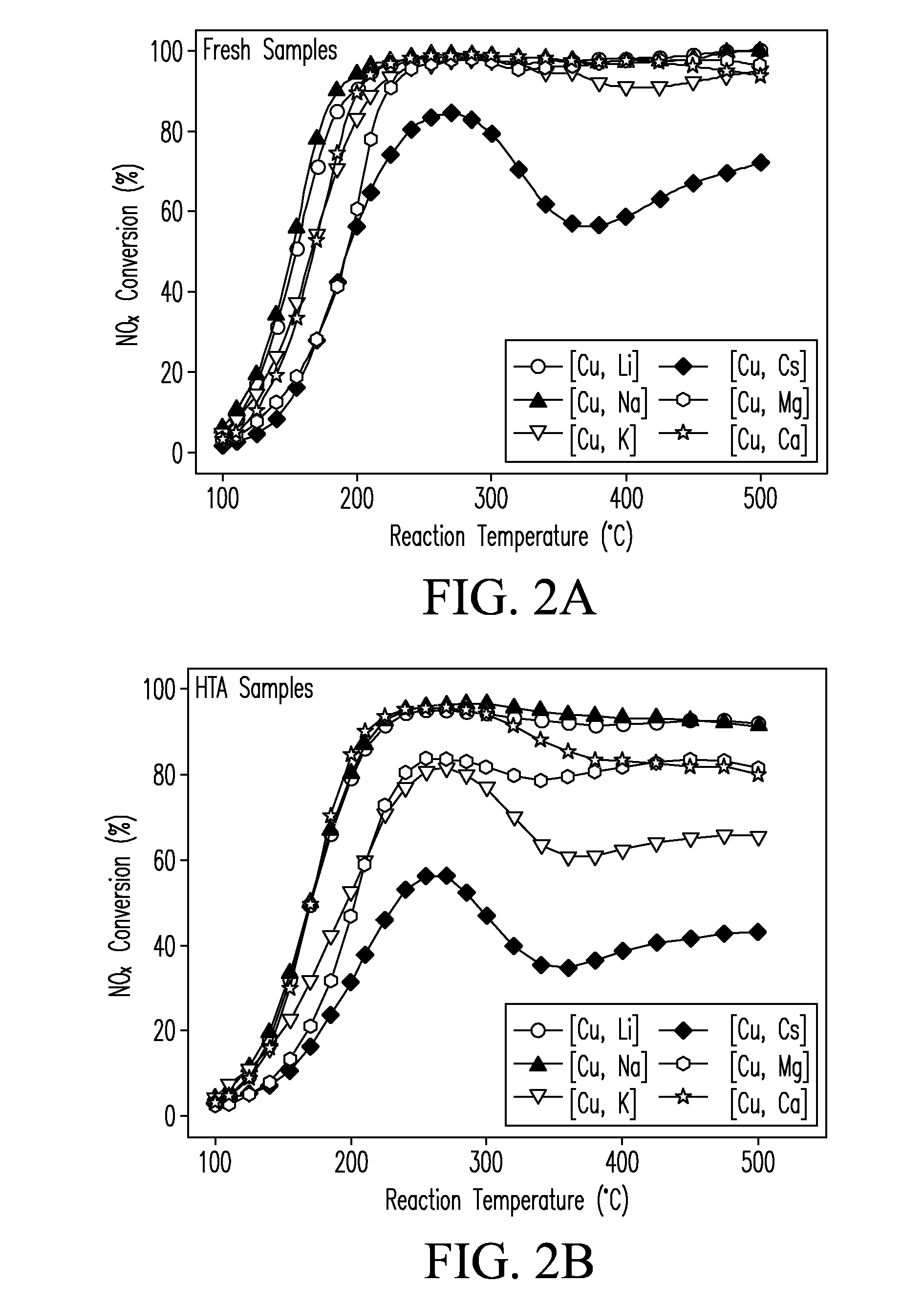
Cu-exchanged zeolite catalysts with a chabazite structure containing selected concentrations of alkali ions or alkaline-earth ions and a lower concentration of (Cu) ions are described and a sequential process for making. Catalysts of the present invention reduce light-off temperatures providing enhanced low-temperature conversion of NOx gases. Catalysts of the present invention also exhibit high selectivity values compared to conventional NOx reduction catalysts.
Owner:BATTELLE MEMORIAL INST
Synthesis of molecular sieves having the chabazite framework type and their use in the conversion of oxygenates to olefins
The synthesis of a crystalline aluminophosphate or silicoaluminophosphate molecular sieve having a chabazite-type framework type is conducted in the presence of an organic directing agent having the formula (I)[R1R2R3N—R4]+X− (I)wherein R1, R2 and R3 are independently selected from the group consisting of alkyl groups having from 1 to 3 carbon atoms and hydroxyalkyl groups having from 1 to 3 carbon atoms; R4 is selected from the group consisting of 4- to 8-membered cycloalkyl groups, optionally substituted by 1 to 3 alkyl groups having from 1 to 3 carbon atoms; 4- to 8-membered heterocyclic groups having from 1 to 3 heteroatoms, said heterocyclic groups being optionally substituted by 1 to 3 alkyl groups having from 1 to 3 carbon atoms and the heteroatoms in said heterocyclic groups being selected from the group consisting of O, N, and S; and aromatic groups optionally substituted by 1 to 3 alkyl groups, said alkyl groups having from 1 to 3 carbon atoms; and X− is an anion.
Owner:EXXONMOBIL CHEM PAT INC
Method for synthesizing zeolite molecular sieve with chabasite structure
ActiveCN106145138ABroaden the chemical compositionExpand the scope ofCrystalline aluminosilicate zeolitesMolecular sieveSeed crystal
The invention provides a method for synthesizing a zeolite molecular sieve with a chabasite structure. The preparation method includes the step of adding organic weak base to obtain the aluminosilicate zeolite molecular sieve with the chabasite structure without adding organic template agents or chabasite seed crystals in chabasite synthesizing reaction under hydrothermal conditions. The molar ratio of framework silicon-aluminum atoms ranges from 4 to 40, and reaction raw materials are prepared according to the molar ratio of Na2O:K2O:the organic weak base Al2O3:SiO2:H2O=(1-20):(2-30):(0.05-8.0):1:(8-80):(150-800). According to the method, the zeolite molecular sieve with the chabasite structure can be stably synthesized without the template agents, fluorinated compounds and the seed crystals.
Owner:北方稀土华凯高科技河北有限公司
Synthesis Of Chabazite Structure-Containing Molecular Sieves And Their Use In The Conversion Of Oxygenates To Olefins
In a method of synthesizing a silicoaluminophosphate or aluminophosphate molecular sieve comprising a CHA framework-type material, a synthesis mixture is provided comprising a source of aluminum, a source of phosphorus, optionally a source of silicon and at least one organic template of formula (I):[R1R2R3R4N]+ X− (I)wherein each of R1, R2, R3, and R4 is independently an acyclic alkyl group having at least one carbon atom, the total number of carbon atoms in said alkyl groups R1, R2, R3, and R4 is greater than 8 but less than 16, and X− is an anion. The synthesis mixture can then be crystallized to produce the desired molecular sieve.
Owner:EXXONMOBIL CHEM PAT INC
Adsorbent Zeolitic Composition, Its Method Of Preparation And Its Use For Removing H2o And/Or Co2 And/Or H2s Contained In Gas Or Liquid Mixtures
ActiveUS20070214959A1Improve the immunityStrong water absorptionSemi-permeable membranesHydrogenAlcoholThiol
The present invention relates to zeolitic compositions of at least one A, X, Y zeolite and / or chabazite and at least one clinoptilolite type of zeolite. These zeolitic compositions can be used in adsorption methods for removing H2O and / or CO2 and / or H2S present in gas or liquid mixtures, particularly for purifying natural gas, acid gases, alcohols and mercaptans.
Owner:ARKEMA FRANCE SA
Hydrothermally stable, low-temperature NOx reduction NH3-SCR catalyst
A catalyst composition includes a heterobimetallic zeolite characterized by a chabazite structure loaded with copper ions and at least one trivalent metal ion other than Al3+. The catalyst composition decreases NOx emissions in diesel exhaust and is suitable for operation in a catalytic converter.
Owner:UT BATTELLE LLC
Cu-Mn bimetallic composite type low-temperature denitration catalyst and preparation method thereof
InactiveCN105854932ALow starting temperatureLower the temperature of the denitrification reactionMolecular sieve catalystsMolecular sieveActive component
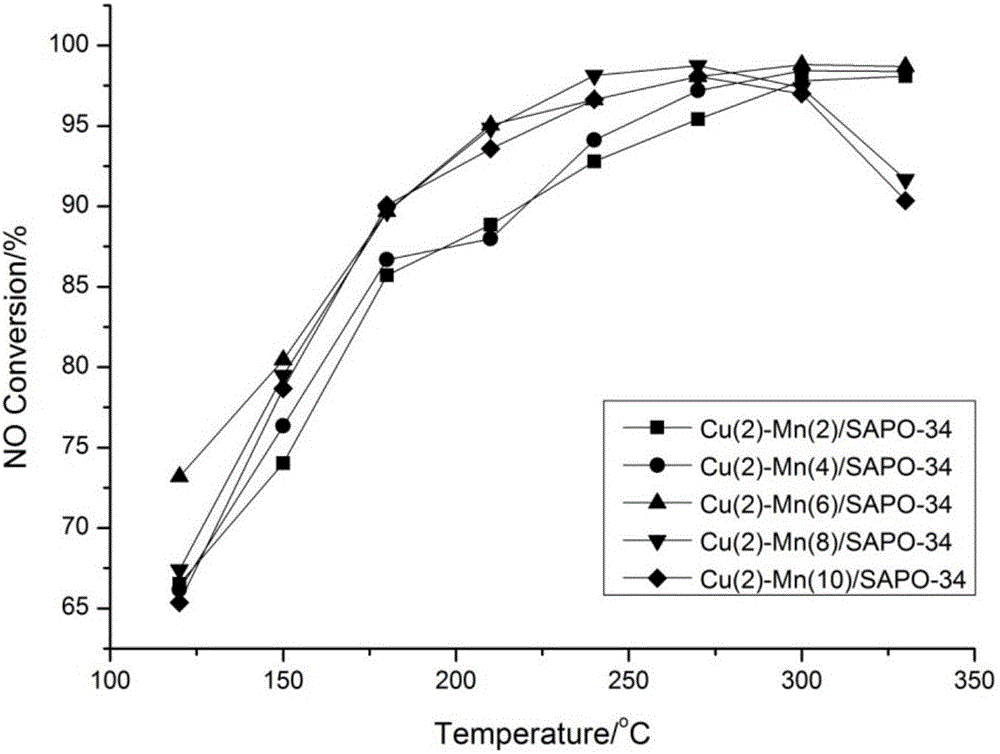
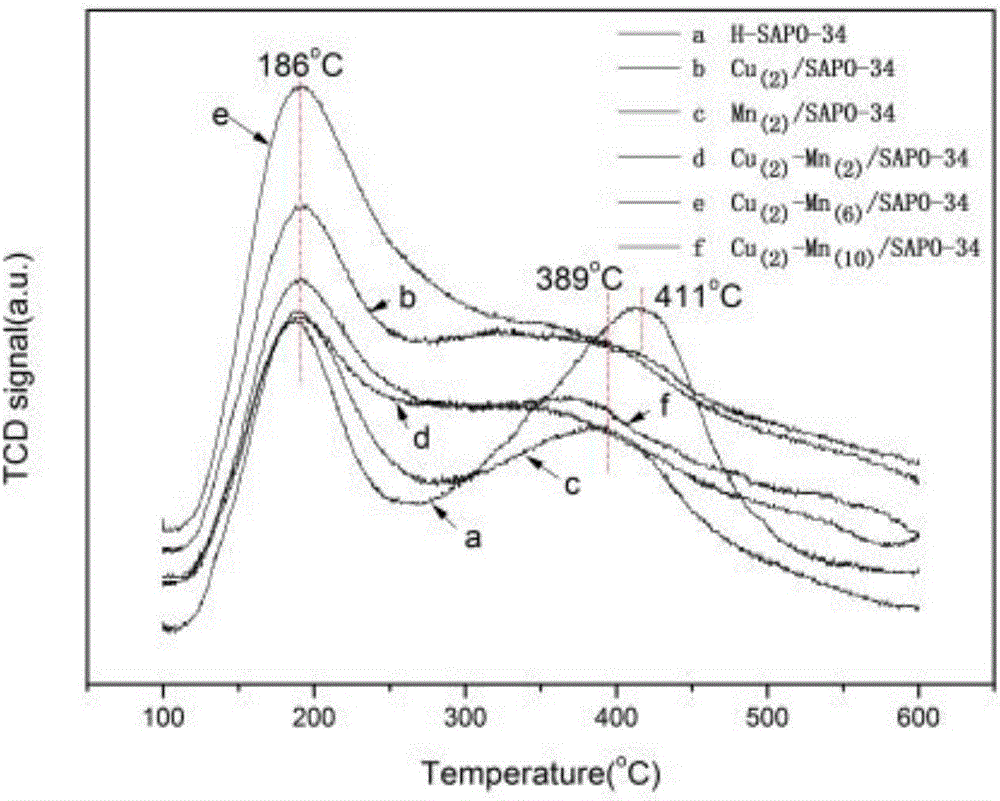
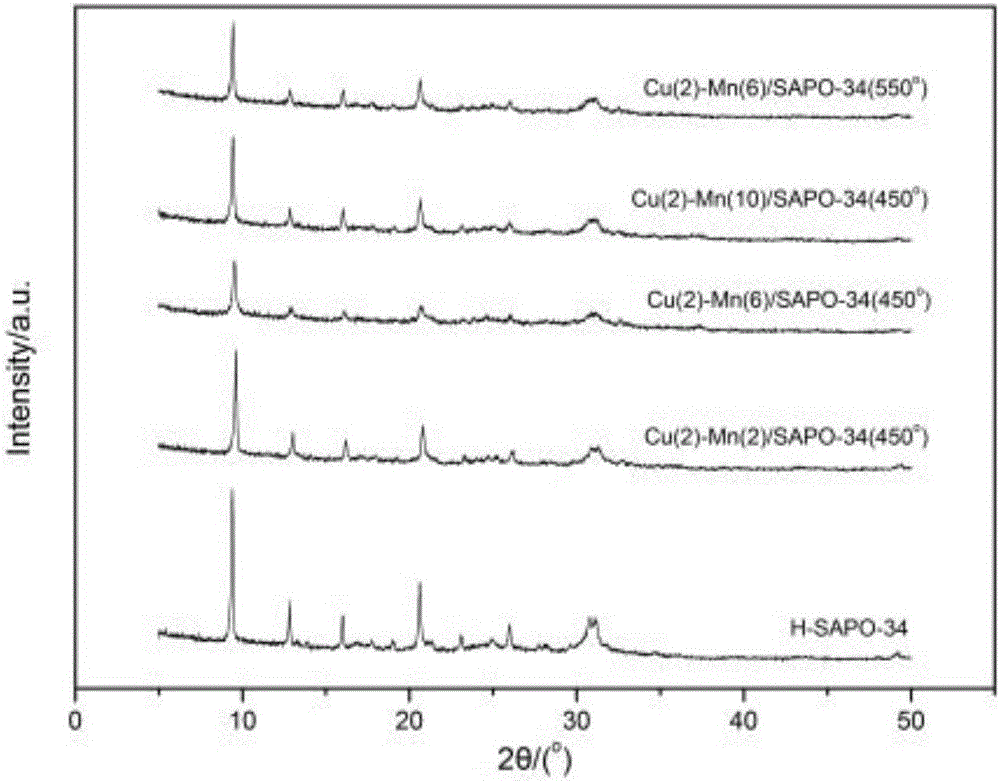
The invention discloses a Cu-Mn bimetallic composite type low-temperature denitration catalyst and a preparation method thereof. The catalyst is prepared by using an immersion method. A chabasite molecular sieve H-SAPO-34 is taken as a carrier, and active components are composite oxides of transition metals Cu and Mn, wherein the weight percentages of the active components Cu and Mn are 2% to 10% respectively. According to the SCR catalyst disclosed by the invention, compared with a Cu-based or Mn-based catalyst of a single component, the denitration efficiency and heat stability of the catalyst are improved obviously by using a bimetallic synergistic effect, and the active temperature window of the catalyst is effectively widened; at the temperature of 180-350 DEG C, the conversion rate of NO can reach 90% or more.
Owner:SOUTHEAST UNIV
Chabazite zeolite membrane having pore size controlled by using chemical vapor deposition and method of preparing the same
ActiveUS20180326365A1Excellent CO2/N separation performanceReducing defect formedMembranesSemi-permeable membranesGas phaseNitrogen
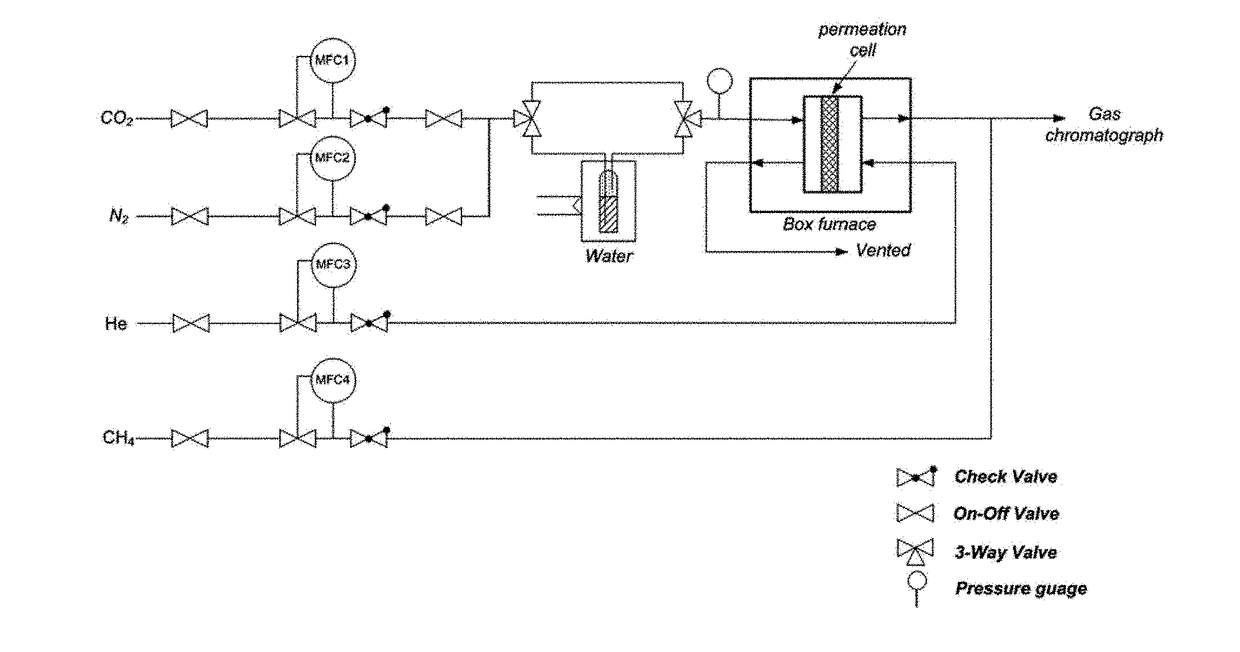
The present invention relates to a chabazite zeolite membrane with a controlled pore size and a production method thereof, wherein the sizes of pore space and pore mouth of the chabazite zeolite membrane are finely controlled through chemical vapor deposition. Through the chemical vapor deposition, defects present in the chabazite zeolite membrane are eliminated, and the pore size is effectively controlled. Thus, unlike hydrophilic membranes showing excellent CO2 / N2 separation performance under a dry condition, the chabazite zeolite membrane with a controlled pore size according to the present invention has a hydrophobic surface, and thus can maintain excellent CO2 / N2 separation performance even under a wet condition. Accordingly, the chabazite zeolite membrane of the present invention can effectively capture carbon dioxide from nitrogen under various environmental conditions.
Owner:KOREA UNIV RES & BUSINESS FOUND
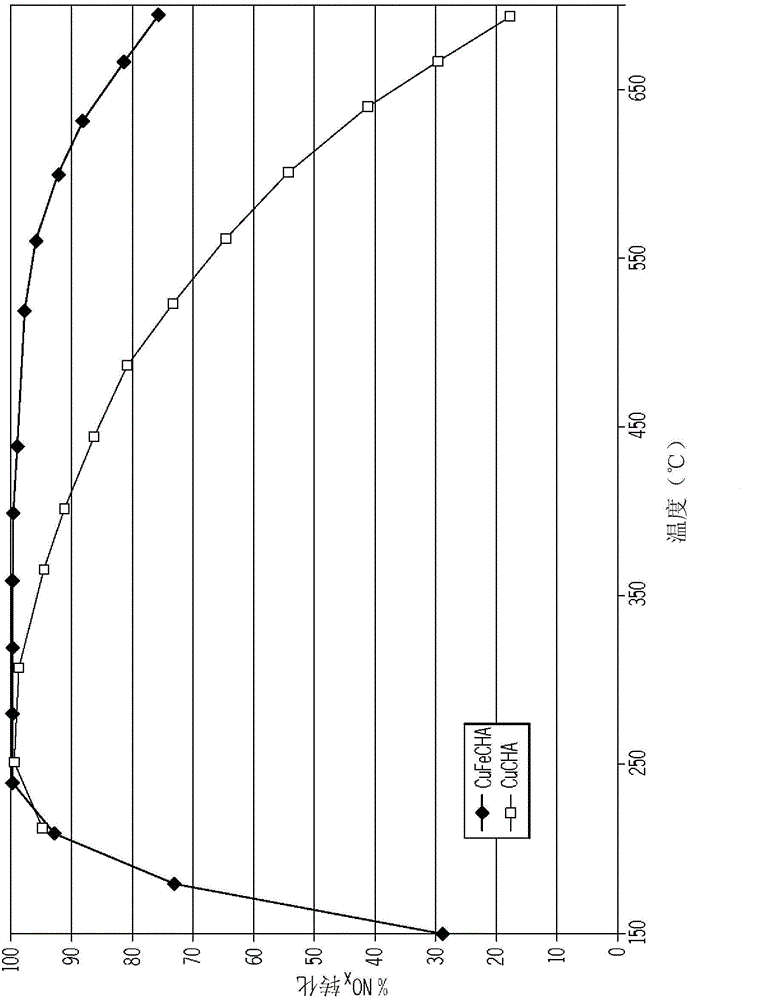
IRON AND COPPER-CONTAINING CHABAZITE ZEOLITE CATALYST FOR USE IN NOx REDUCTION
InactiveCN104971766AMolecular sieve catalystsInternal combustion piston enginesNitrogen oxidesIon exchange
A chabazite (CHA) zeolite catalyst containing iron and copper is provided as an SCR catalyst for reducing nitrogen oxides (NOx) from vehicle engine exhausts. The catalyst is formed by incorporating iron during synthesis of the chabazite zeolite, followed by incorporating copper in an ion-exchange step. The resulting catalyst reduces nitrogen oxides over a wide range of temperatures from about 200° C. to about 700° C.
Owner:FORD GLOBAL TECH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com