Preparation method for nickel-manganese-cobalt anode material of lithium ion battery
A positive electrode material, nickel-manganese-cobalt technology, applied in battery electrodes, electrical components, circuits, etc., can solve physical indicators such as difficult control of particle size and morphology characteristics, inability to form a homogeneous eutectic, and unstable batch product quality and other problems to achieve high tap density, stable cycle performance and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
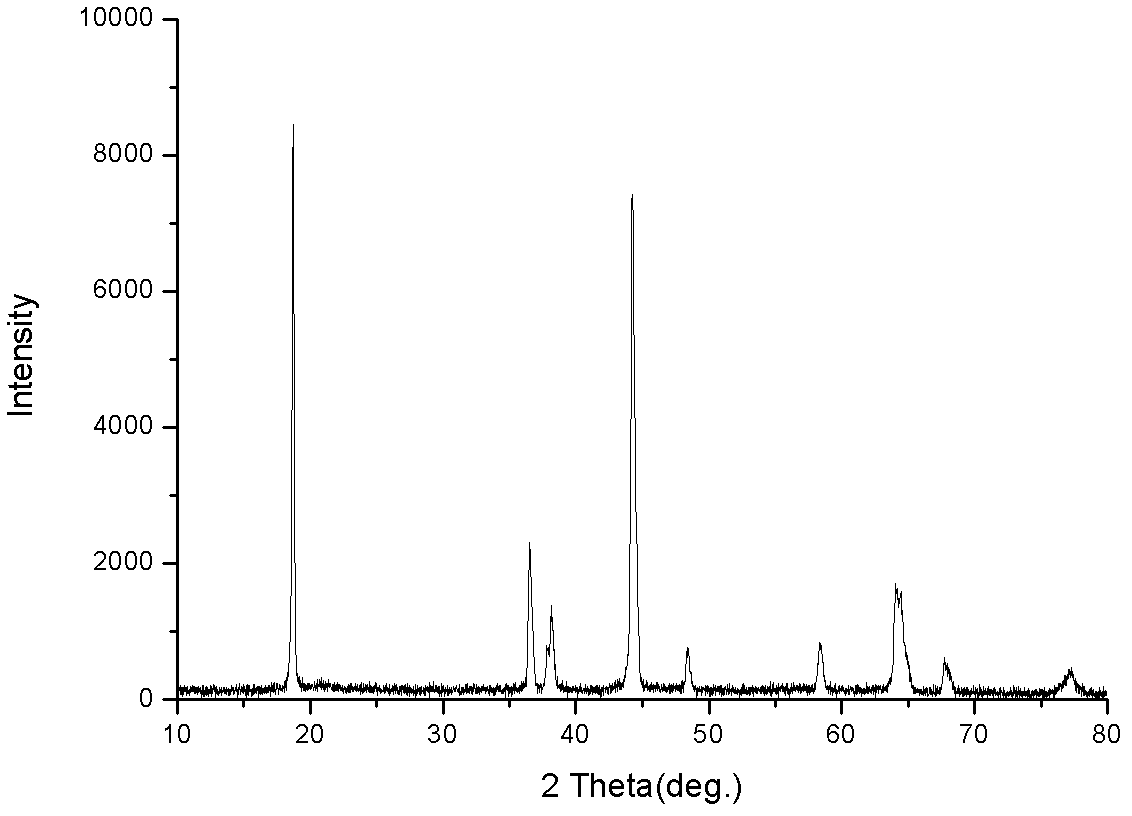
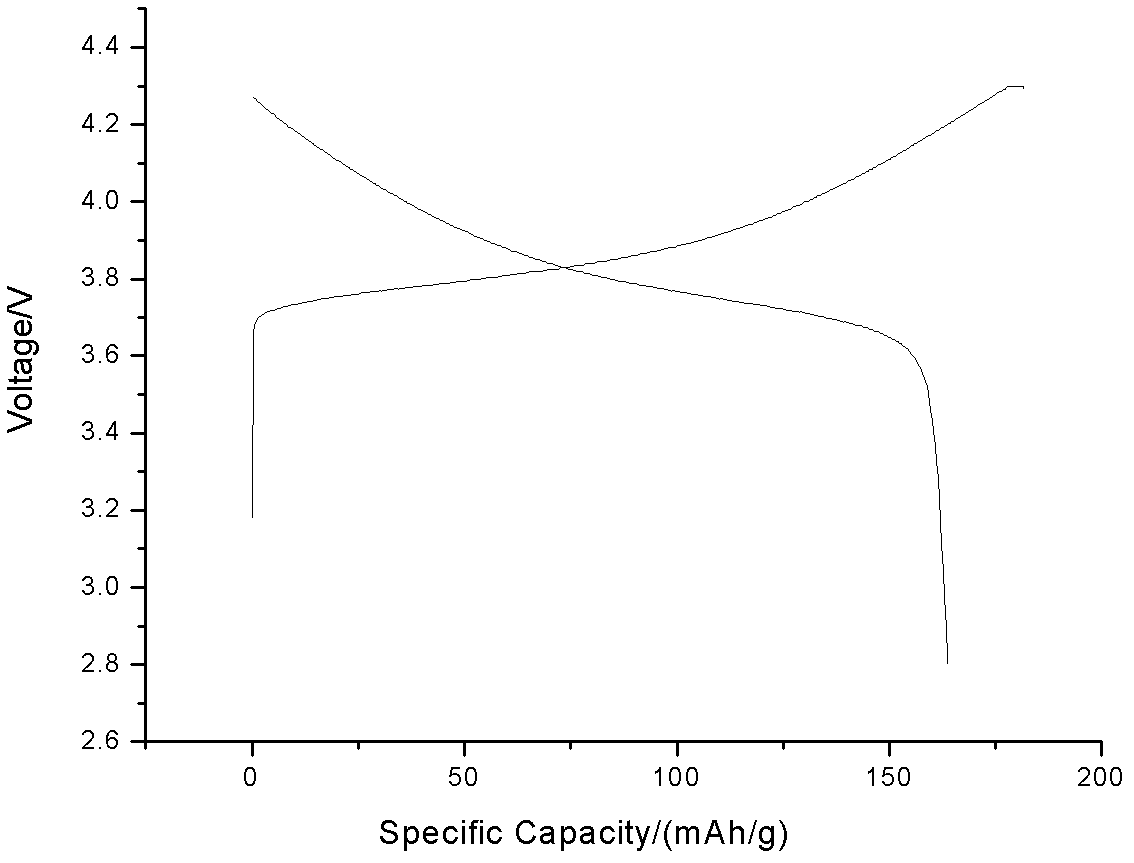
Image
Examples
Embodiment 1
[0032](1) Dissolve equimolar amounts of manganese sulfate (MnSO4·H2O), cobalt sulfate (CoSO4·7H2O), and nickel sulfate (NiSO4·6H2O) in deionized water to form a mixed solution of 1 mol·L. Water Mixed solution of 2 mol L-1 NaOH and 0.48 mol L-1 ammonia water, the volume ratio of the mixed solution and ammonia water is 1:4;
[0033] (2) With a constant current pump, the above-mentioned nickel-manganese-cobalt ion mixed solution and ammonia solution mixed solution are added respectively in the reaction vessel of the nitrogen atmosphere of high-speed stirring, and NaOH solution is added dropwise to adjust the pH to be 12, aged for 10h, and controlled aging The pH of the chemicalization process is 12. The precipitate obtained in the above steps is fully washed, filtered, and dried in vacuum at 100~110°C for 12 hours to obtain the Ni1 / 3Co1 / 3Mn1 / 3(OH)2 precursor;
[0034] (3) Weigh 30 g of nickel-manganese-cobalt hydroxide, 12.11 g of lithium carbonate, add 0.2105 g of alumina ball m...
Embodiment 2
[0038] According to the molar ratio Ni:Co:Mn=1:1:1, prepare a mixed aqueous solution of nickel sulfate, manganese sulfate, and cobalt sulfate with a total concentration of 1.3mol / L, prepare a NaOH solution with a concentration of 4mol / L, and prepare a concentration of 0.66mol / L of ammonia solution. The volume ratio of the mixed solution and ammonia water is 1:3.5.
[0039] Add the above-mentioned nickel-manganese-cobalt ion mixed solution and ammonia solution into the reactor to stir and react with a constant-current pump, add NaOH solution dropwise to adjust the pH to 14, age for 18 hours, and control the pH of the aging process to be 14. The precipitate was fully washed, filtered, and dried in vacuum at 100-110°C for 12 hours to obtain the Ni1 / 3Co1 / 3Mn1 / 3(OH)2 precursor.
[0040] Weigh 20 g of nickel-manganese-cobalt hydroxide, 8.235 g of lithium carbonate, add 0.2258 g of chromium oxide ball mill and mix, place in an air atmosphere muffle furnace, and then heat up to Ins...
Embodiment 3
[0044] According to the molar ratio Ni:Co:Mn=1:1:1, prepare a mixed aqueous solution of nickel chloride, manganese chloride, and cobalt chloride with a total concentration of 1.1mol / L, prepare a NaOH solution with a concentration of 6mol / L, and prepare a concentration of It is a 0.24mol / L ammonia solution, and the volume ratio of the mixed solution to the ammonia solution is 1:3.
[0045] Add the above-mentioned nickel-manganese-cobalt ion mixed solution and ammonia solution into the reactor to stir and react with a constant flow pump, add NaOH solution dropwise to adjust the pH to 13, age for 18 hours, and control the pH of the aging process to be 13, the above steps are obtained The precipitate was fully washed, filtered, and dried in vacuum at 100-110°C for 12 hours to obtain Ni 1 / 3 co 1 / 3 mn 1 / 3 (OH) 2 Precursor.
[0046] Weigh 60g of nickel-manganese-cobalt hydroxide, 25.43g of lithium carbonate, add 0.68334g of zinc oxide ball mill and mix, place in an oxygen atmosph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com