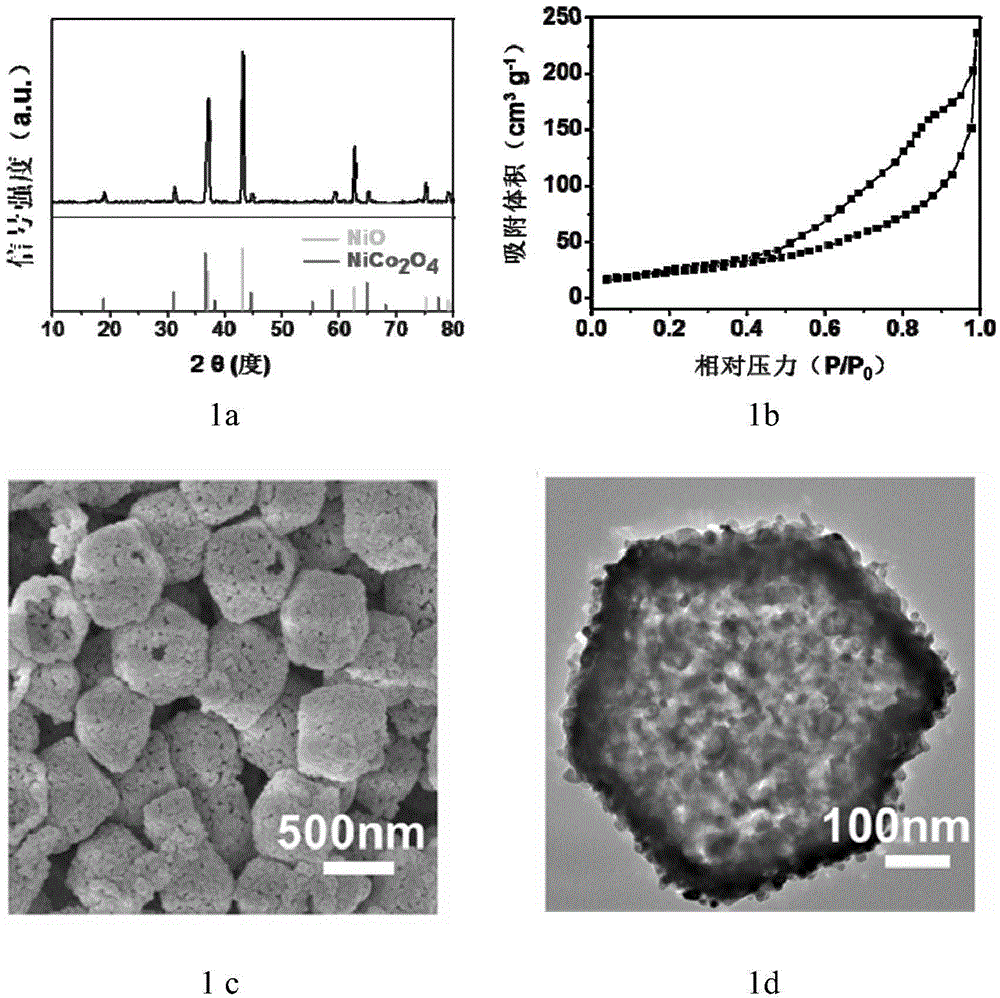
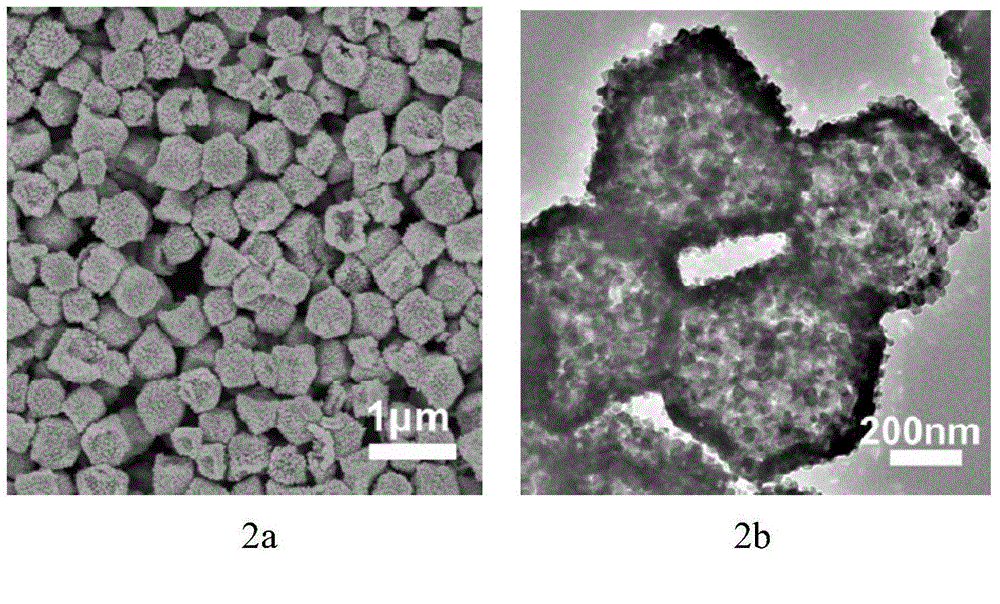
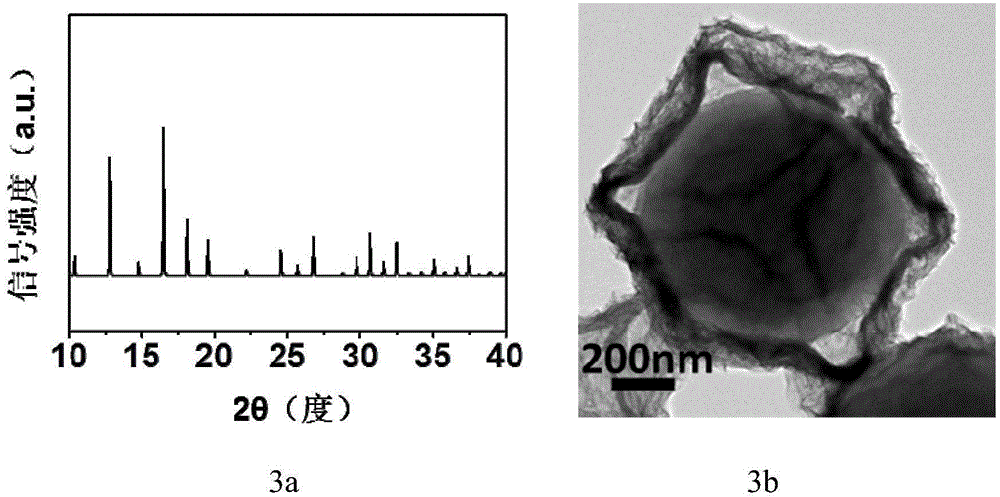
Preparation method of hollow nickel cobaltate nano polyhedron
A polyhedron, nickel cobalt oxide technology, applied in nanotechnology, chemical instruments and methods, cobalt compounds, etc., can solve the problems of poor product dispersion, large product particle size, long reaction time, etc., and achieve easy preparation, uniform particles, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Dissolve 0.146 grams of cobalt nitrate hexahydrate and 0.328 grams of dimethylimidazole in 25 milliliters of anhydrous methanol respectively, then mix the two solutions fully, let stand for 20 hours, collect the precipitate, centrifuge several times, and wash with absolute ethanol Afterwards, it was dried in a vacuum oven at 60°C for 12 hours to obtain the metal-organic framework complex ZIF-67.
[0044] Add 0.150 grams of nickel nitrate to 25 milliliters of absolute ethanol, mix well, and form a clear solution, then add the metal-organic framework complex ZIF-67 powder and mix thoroughly, place in an oven at 90°C, and keep the temperature for 1 hour . After the reaction is completed, cool to room temperature, collect the precipitate, wash the obtained product with ethanol, centrifuge, discard the solvent, and dry it in vacuum at 60°C to obtain a nickel-cobalt hydroxide precursor (ie, a hollow polyhedron of nickel-cobalt hydroxide).
[0045] The precursor is heated at ...
Embodiment 2
[0047] Dissolve 0.728 grams of cobalt nitrate hexahydrate and 0.547 grams of dimethylimidazole in 25 milliliters of anhydrous methanol respectively, and then mix the two solutions fully and let stand for 30 hours to collect the precipitate. The resulting product is centrifuged several times and washed with anhydrous After washing with ethanol, dry in a vacuum oven at 60°C for 12 hours to obtain the metal organic framework complex ZIF-67.
[0048] Add 0.100 g of nickel nitrate into 25 ml of anhydrous methanol, mix well to form a clear solution, add the above metal organic framework complex ZIF-67 powder and mix well, place in an oven at 80°C, and keep the temperature for 2 hours . After the reaction is completed, cool to room temperature, collect the precipitate, wash the obtained product with acetone, centrifuge, discard the solvent, and dry in vacuum at 60°C to obtain a nickel-cobalt hydroxide precursor (ie, a hollow polyhedron of nickel-cobalt hydroxide).
[0049] The precu...
Embodiment 3
[0051] Dissolve 0.249g of cobalt nitrate hexahydrate and 0.328g of dimethylimidazole in 25mL of anhydrous methanol respectively, and then mix the two solutions fully, and let stand for 24 hours to collect the precipitate. The obtained product is centrifuged several times and washed with absolute ethanol After washing, it was placed in a vacuum drying oven at 60° C. for 12 hours to obtain the metal-organic framework complex ZIF-67.
[0052] Add 0.050 g of nickel nitrate to 25 ml of absolute ethanol, mix well to form a clear solution, add the above metal organic framework complex ZIF-67 powder and mix well, place in an oven at 90°C, and keep the temperature for 1 hour . After the reaction is completed, cool to room temperature, collect the precipitate, wash the obtained product with ethanol, centrifuge, discard the solvent, and dry it in vacuum at 60°C to obtain a nickel-cobalt hydroxide precursor (ie, a hollow polyhedron of nickel-cobalt hydroxide). Such as image 3 The X-ray...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com