Method for preparing lithium carbonate from lepidolite through sulfuric acid roasting method
A technology of sulfuric acid roasting and lepidolite, which is applied in the direction of lithium carbonate; high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
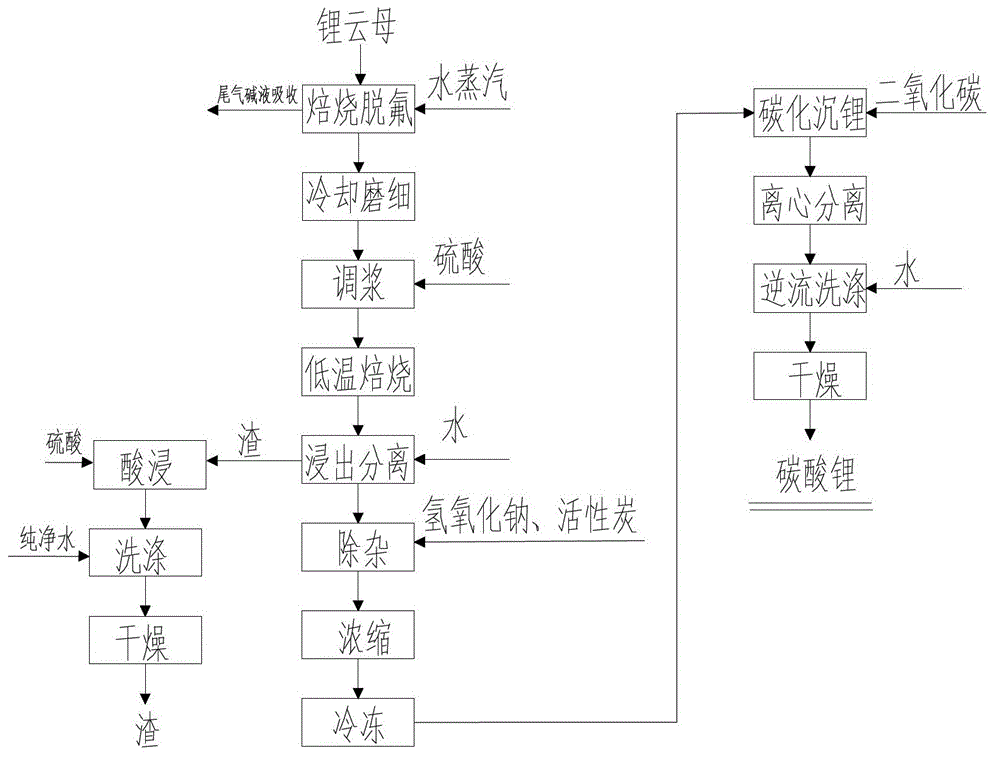
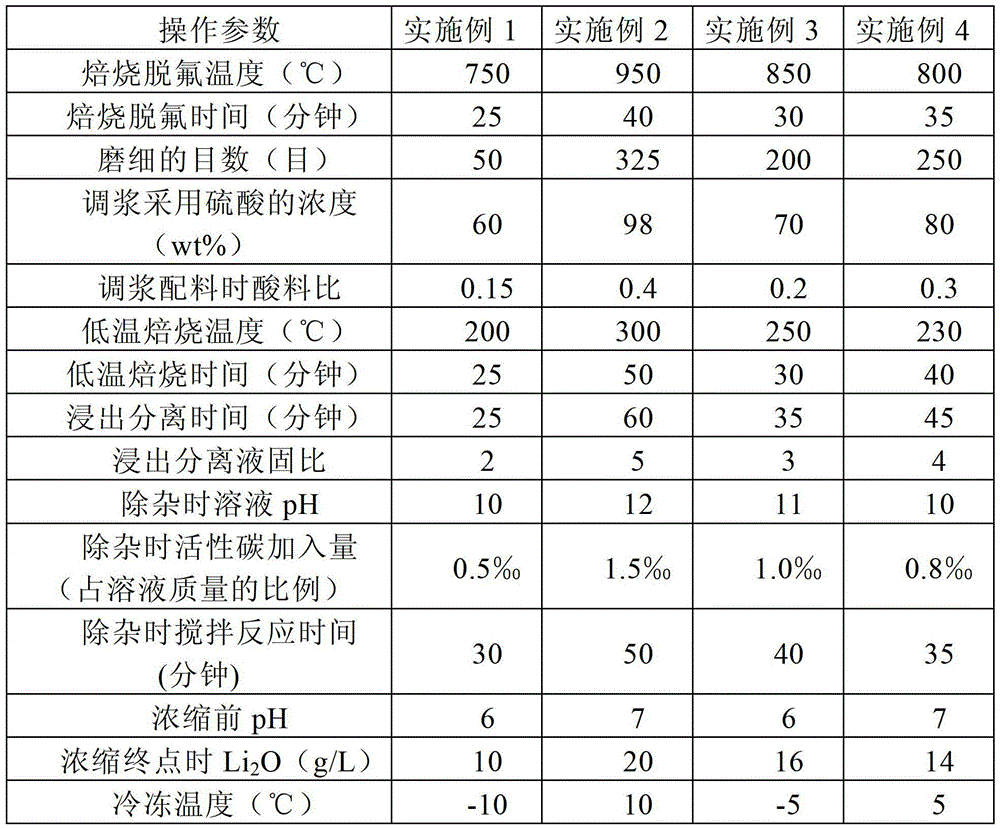
[0057] (1) Roasting defluorination: Roast 1000Kg lepidolite ore at 750°C with water vapor for 25 minutes for defluorination, the flow rate of water vapor is 0.005m 3 / h;
[0058] (2) Cooling and grinding: Cool the lepidolite after roasting in step (1) to room temperature, then grind it to 50 mesh, and analyze and detect Li in lepidolite 2 O content is 3.87%;
[0059] (3) Slurry: 233Kg60% (wt%, which is the mass percentage) of H 2 SO 4 Mix with the 931Kg pulverized lepidolite obtained in step (2) to form a slurry;
[0060] (4) Low-temperature roasting: put 1164Kg of mixed slurry obtained in step (3) in a rotary furnace, and roast at 200°C for 25 minutes to obtain 1028Kg of roasted material;
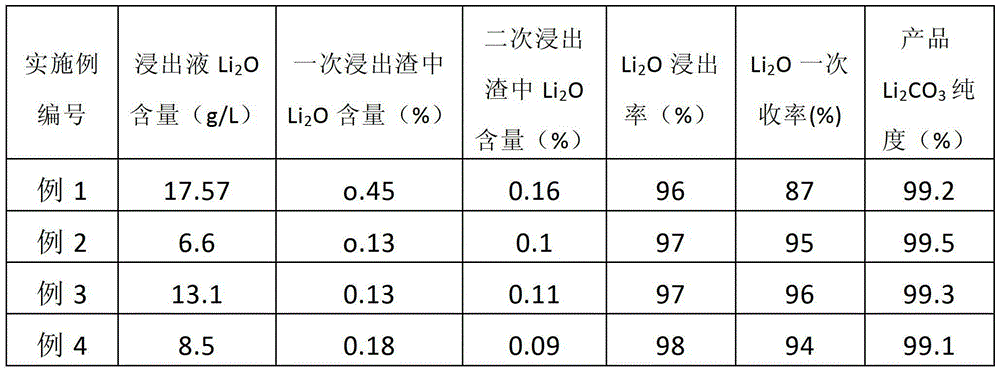
[0061] (5) Leaching and separation: add 2056Kg pure water to the 1028Kg roasted material obtained in step (4), stir at room temperature for 25 minutes, then centrifuge to obtain 1954Kg filtrate and 1130Kg filter residue, filter residue with 60% H 2 SO 4 Mix according to the liquid-so...
Embodiment 2
[0067] (1) Roasting defluorination: Roast 1200Kg lepidolite ore with water vapor at 950°C for 40 minutes for defluorination, the flow rate of water vapor is 0.018m 3 / h;
[0068] (2) Cooling and grinding: Cool the lepidolite after roasting in step (1) to room temperature, then grind it to 325 mesh, and analyze and detect Li in lepidolite 2 O content is 4.29%;
[0069] (3) Slurry: 446Kg mass percentage of 98% H 2 SO 4 Mix with the 1117Kg pulverized lepidolite obtained in step (2) to form a mixed slurry;
[0070] (4) Low-temperature roasting: 1563Kg of mixed slurry obtained in step (3) was placed in a rotary furnace, and roasted at 300°C for 50 minutes to obtain 1532Kg of roasted material;
[0071] (5) Leaching and separation: add 7663Kg of pure water to the 1532Kg roasted material obtained in step (4), stir at room temperature for 60 minutes, and then centrifuge to obtain 7510Kg of filtrate and 1685Kg of filter residue, the filter residue is 98% H 2 SO 4 Mix according to ...
Embodiment 3
[0077] (1) Roasting defluorination: Roast 1500Kg lepidolite ore with water vapor at 850°C for 30 minutes for defluorination, the flow rate of water vapor is 0.018m 3 / h;
[0078](2) Cooling and grinding: Cool the lepidolite after roasting in step (1) to room temperature, then grind it to 200 mesh, and analyze and detect Li in lepidolite 2 O content is 4.13%;
[0079] (3) Mixing: 398Kg70% H 2 SO 4 Mix with the 1396Kg pulverized lepidolite obtained in step (2) to form a mixed slurry;
[0080] (4) Low-temperature roasting: put the 1794Kg mixed slurry obtained in step (3) in a rotary furnace, and roast at 250°C for 30 minutes;
[0081] (5) Leaching and separation: add 4925Kg washing water (washing water in the previous step) to the 1641Kg roasted material obtained in step (4), stir at room temperature for 35 minutes, and then centrifuge to obtain 4761Kg filtrate and 1805Kg filter residue, the filter residue is used 70% H by mass 2 SO 4 Mix according to the liquid-solid mass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com