Clean method for preparing layered double hydroxides
a technology of layered double hydroxide and layered hydroxide, which is applied in the field of clean method for preparing layered double hydroxide, can solve the problems of increasing solubility, difficult to dissolve in water, and low product constant of hydrogen oxide, so as to avoid the use reduce the effect of atom efficiency and avoid the effect of naoh or other materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0024]MgAl—CO3-LDHs were prepared as follows.
[0025]Step A: Total 10 g Mg(OH)2 and Al(OH)3 with a molar ratio of Mg2+ / Al3+=2:1 were put into 90 g of deionized water, and the resulting mixture was transferred to a three-necked flask fitted with a reflux device.
[0026]Step B: The mixture was heated to 100° C. under stirring for four days, while CO2 was fed thereto at a flow rate of 10 ml / min. The resulting slurry product was filtered and dried in air at 70° C. for 8 h to give Mg4Al2(OH)12CO3.4H2O.
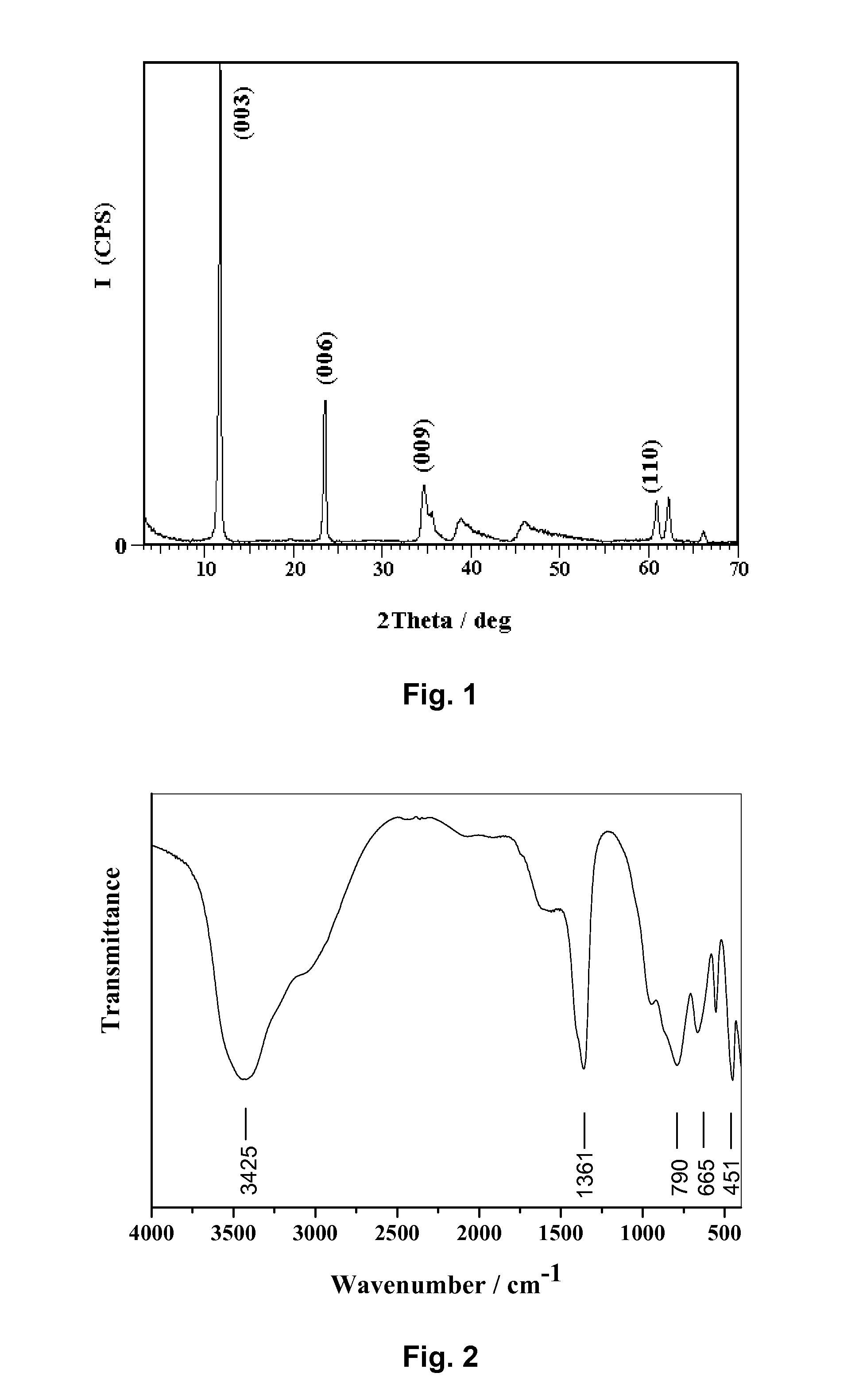
[0027]The powder XRD patterns of the prepared Mg4Al2(OH)12CO3.4H2O were recorded using a Shimadzu XRD-6000 diffractometer, and were shown in FIG. 1. The typical peaks which correspond to the (003), (006), (009) reflections were found at 2θ=11.7°, 23.4°, 34.5°. It could be concluded from the patterns that the samples had a layered crystal structure.
[0028]The infrared spectra of the samples, obtained using a Bruker Vector 22 model Fourier transform infrared spectrometer (FT-IR), were shown in FIG...
example 2
[0029]Ni8Fe2(OH)20(C8H4O4).4H2O was prepared as follows.
[0030]Step A: Total 5 g Ni(OH)2 and Fe(OH)3 with a molar ratio of Ni2+ / Fe3+=4:1 were put into 90 g of deionized water, and the resulting mixture was transferred to a three-necked flask fitted with a reflux device.
[0031]Step B: 0.58 g terephthalic acid was added to the flask. The contents in the flask were heated to 100° C. and reacted for eight days under stirring. After filtration and drying in air at 70° C. for 8 h, Ni8Fe2(OH)20(C8H4O4).4H2O was obtained.
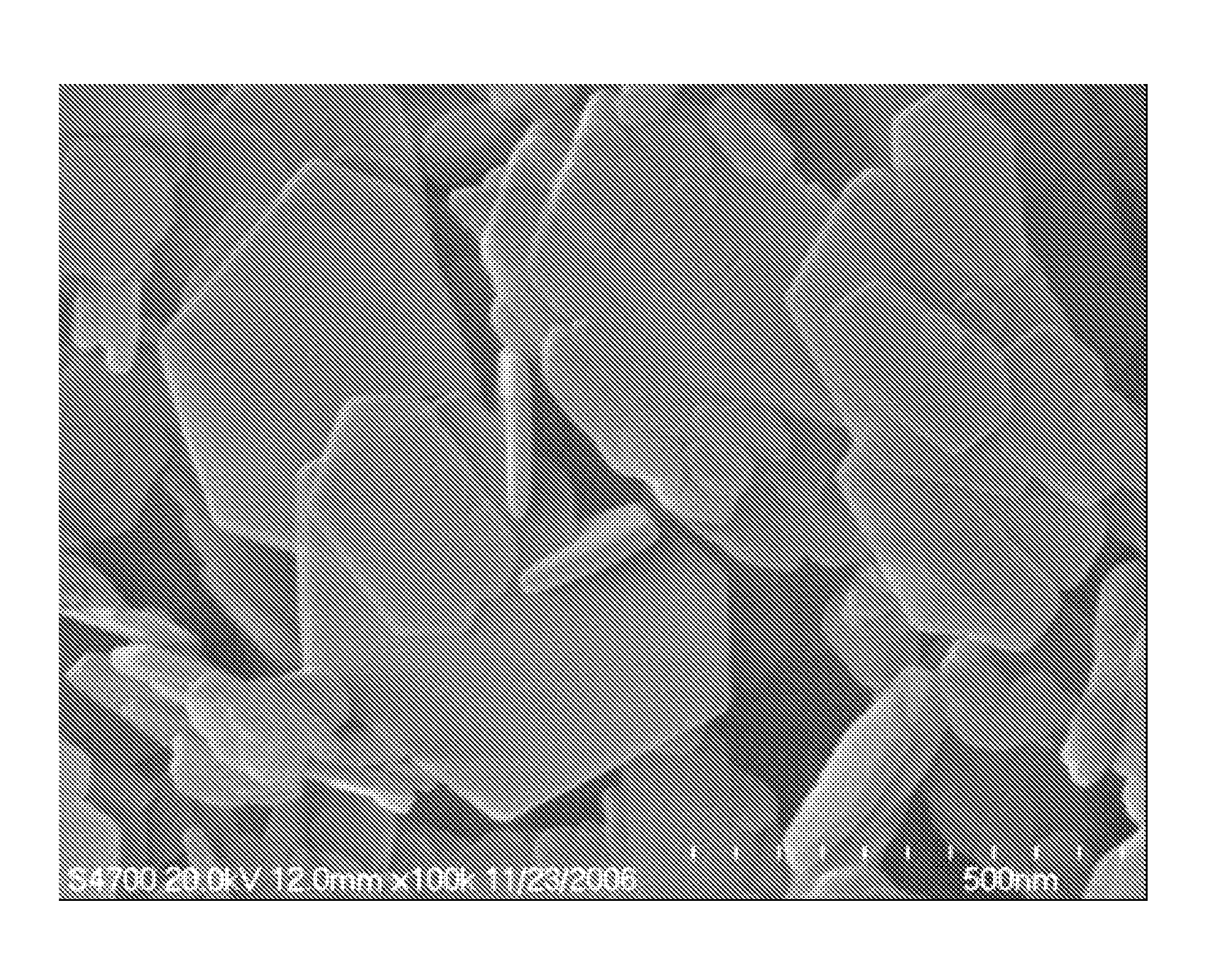
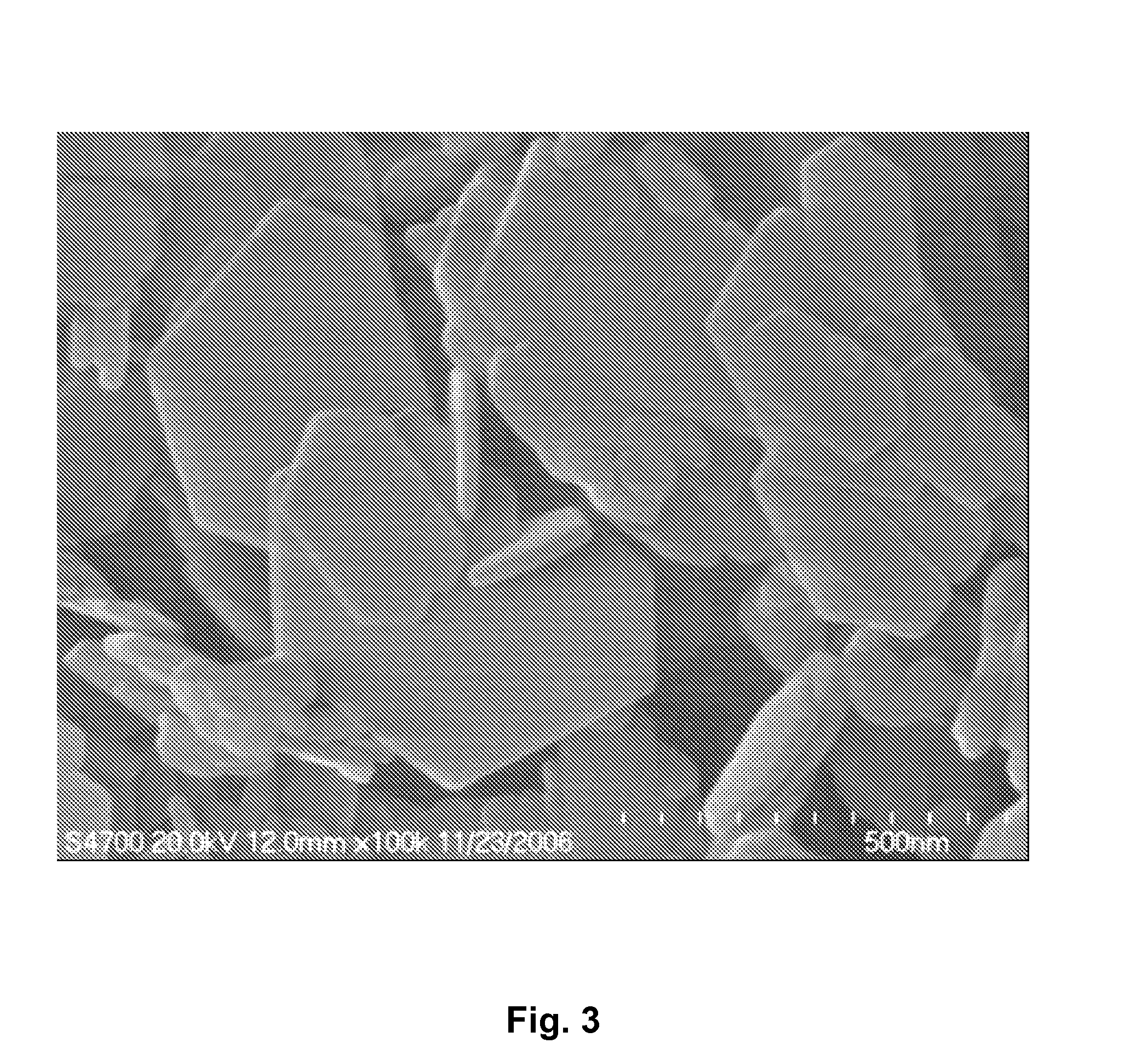
[0032]The TEM image of the product produced in example 2, obtained using a Hitachi S-3500N scanning electron microscope, was shown in FIG. 3. The image reveals a layered hexagonal morphology of LDHs.
example 3
[0033]ZnMg3Al2(OH)12CO3.4H2O was prepared as follows.
[0034]Step A: Total 20 g Zn(OH)2, Mg(OH)2 and Al(OH)3 with a molar ratio of Zn2+ / Mg3+ / Al3+=1:3:2 were put into 80 g of deionized water, and the mixture was transferred to an airtight reactor fitted with a churn-dasher.
[0035]Step B: After adding 40 g of dry ice to the reactor, the system was heated at 150° C. for one day under stirring. The resulting slurry was filtered and dried in air at 70° C. for 8 h to give ZnMg3Al2(OH)12CO3.4H2O.
[0036]Elemental analysis of the product obtained in example 3 was performed on an ICPS-7500 inductively coupled plasma spectrometer. The molar ratio of Zn:Mg:Al was measured as 1:3:2 and no Na+ was found in the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com