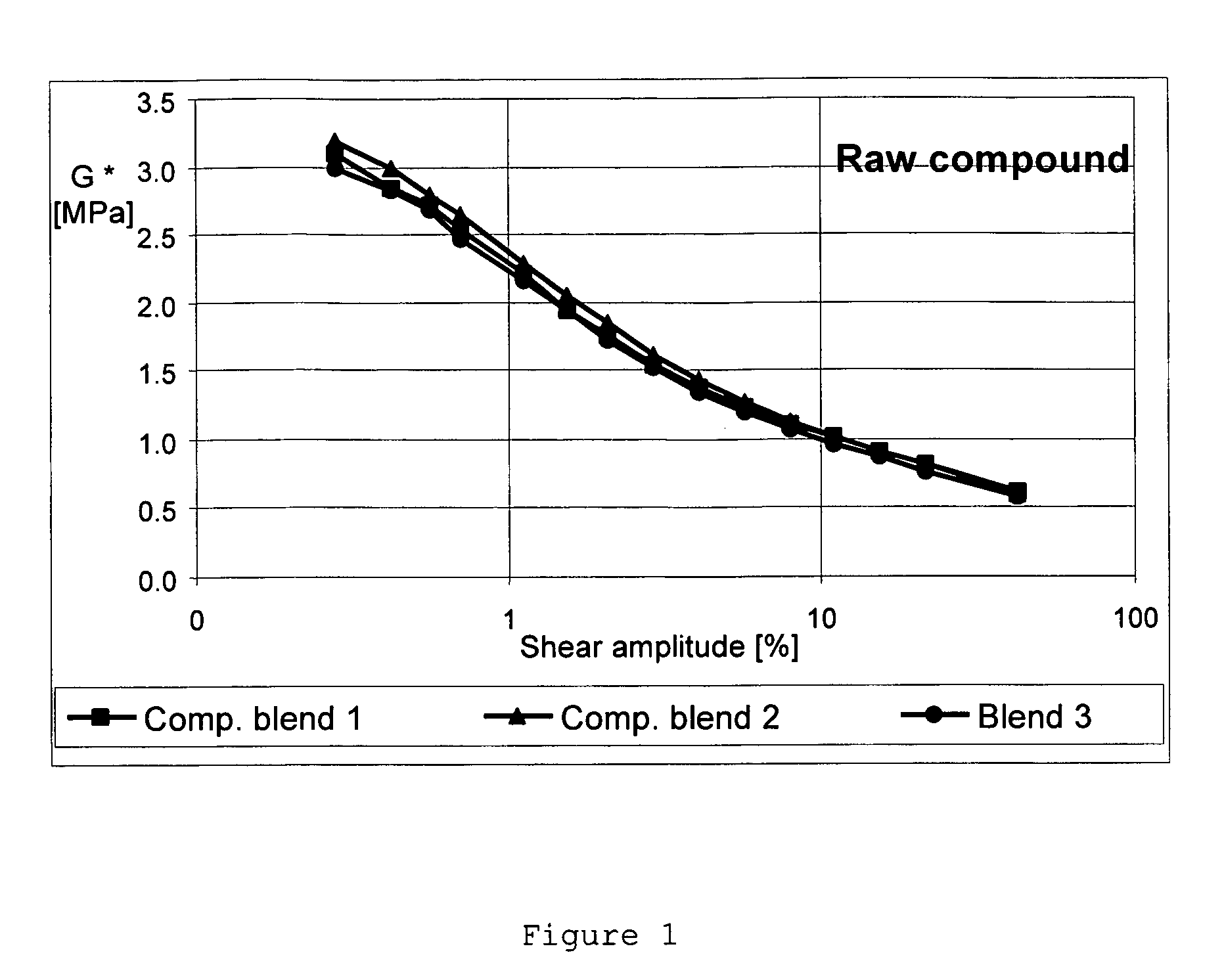
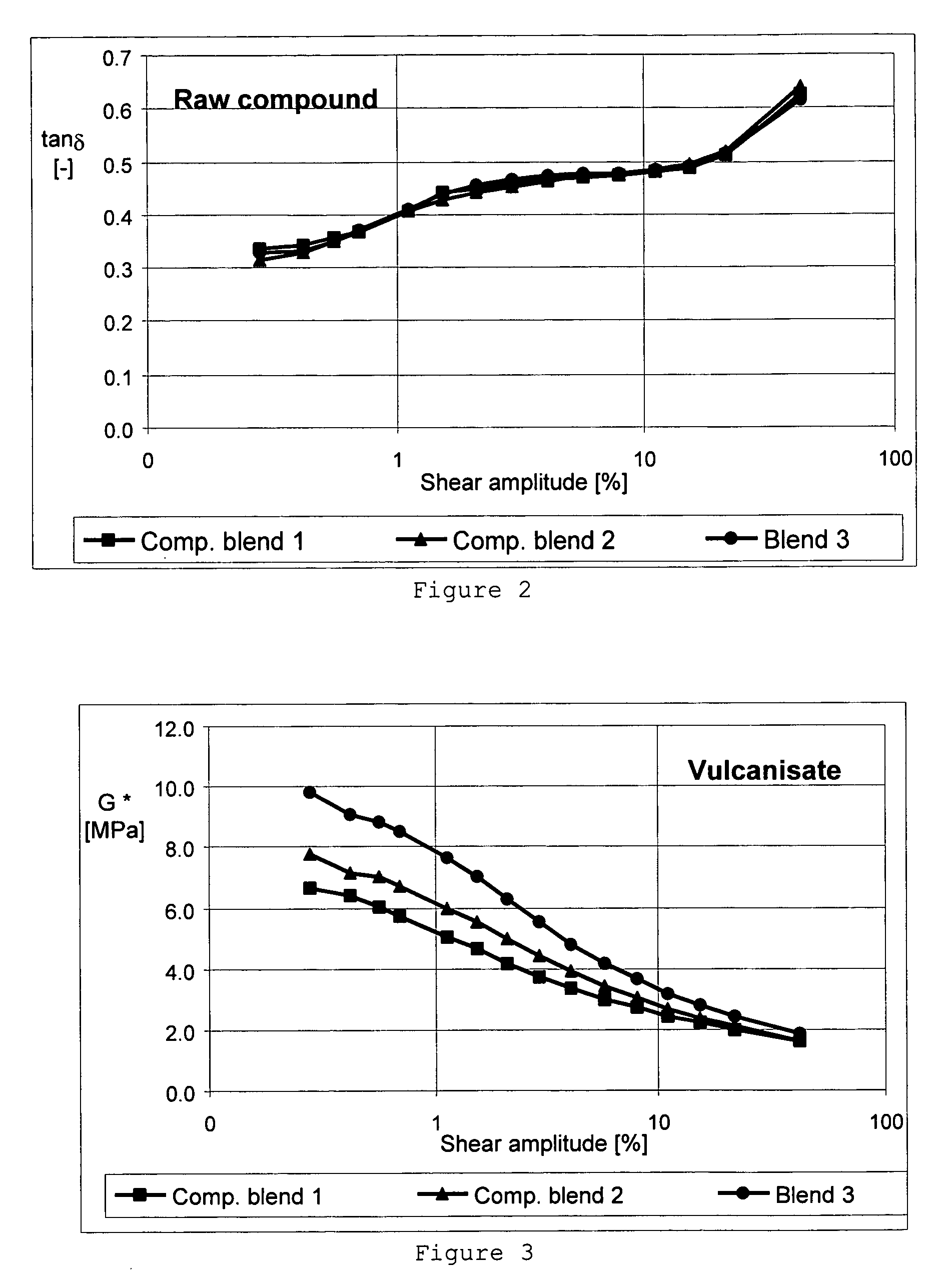
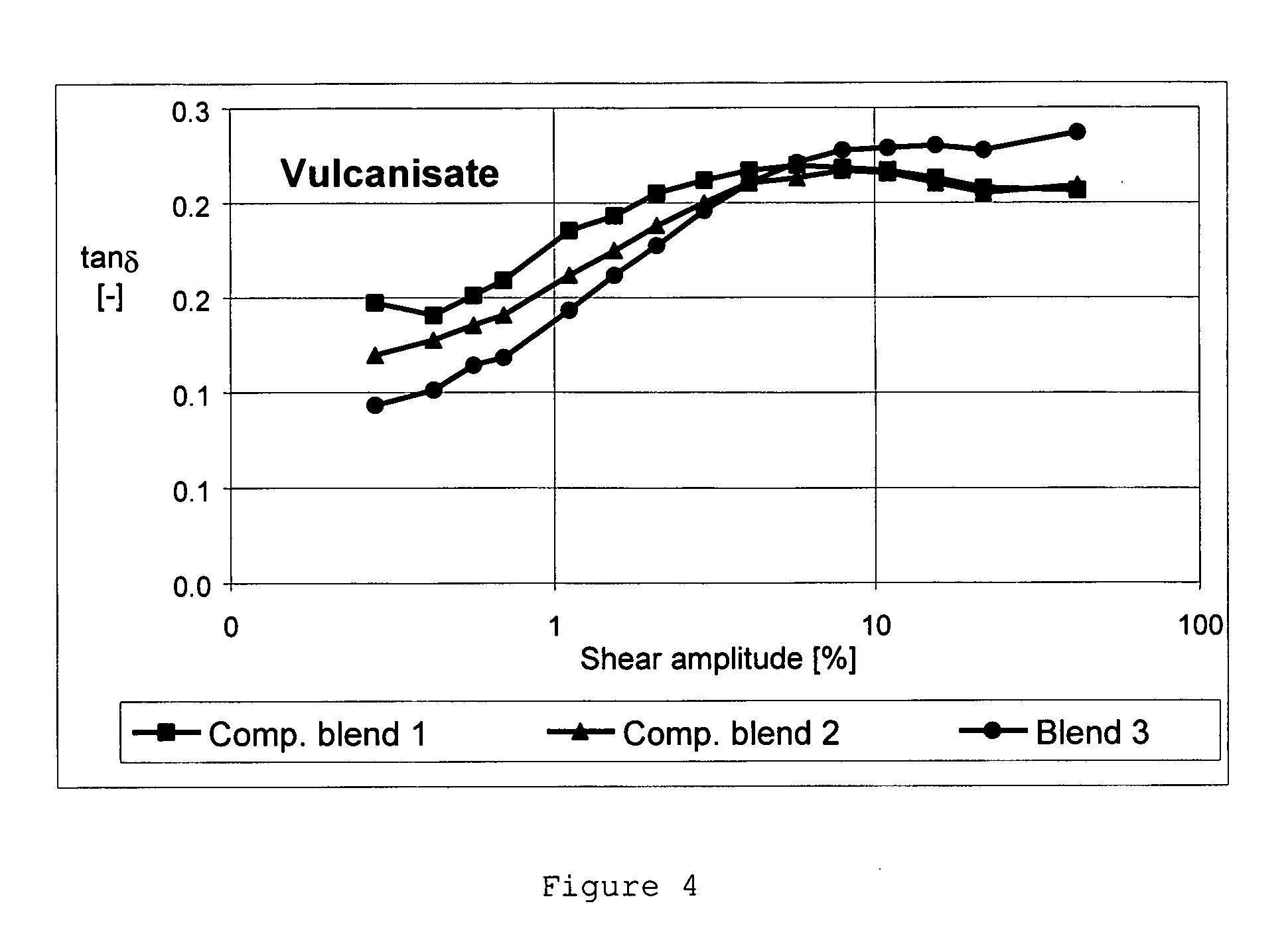
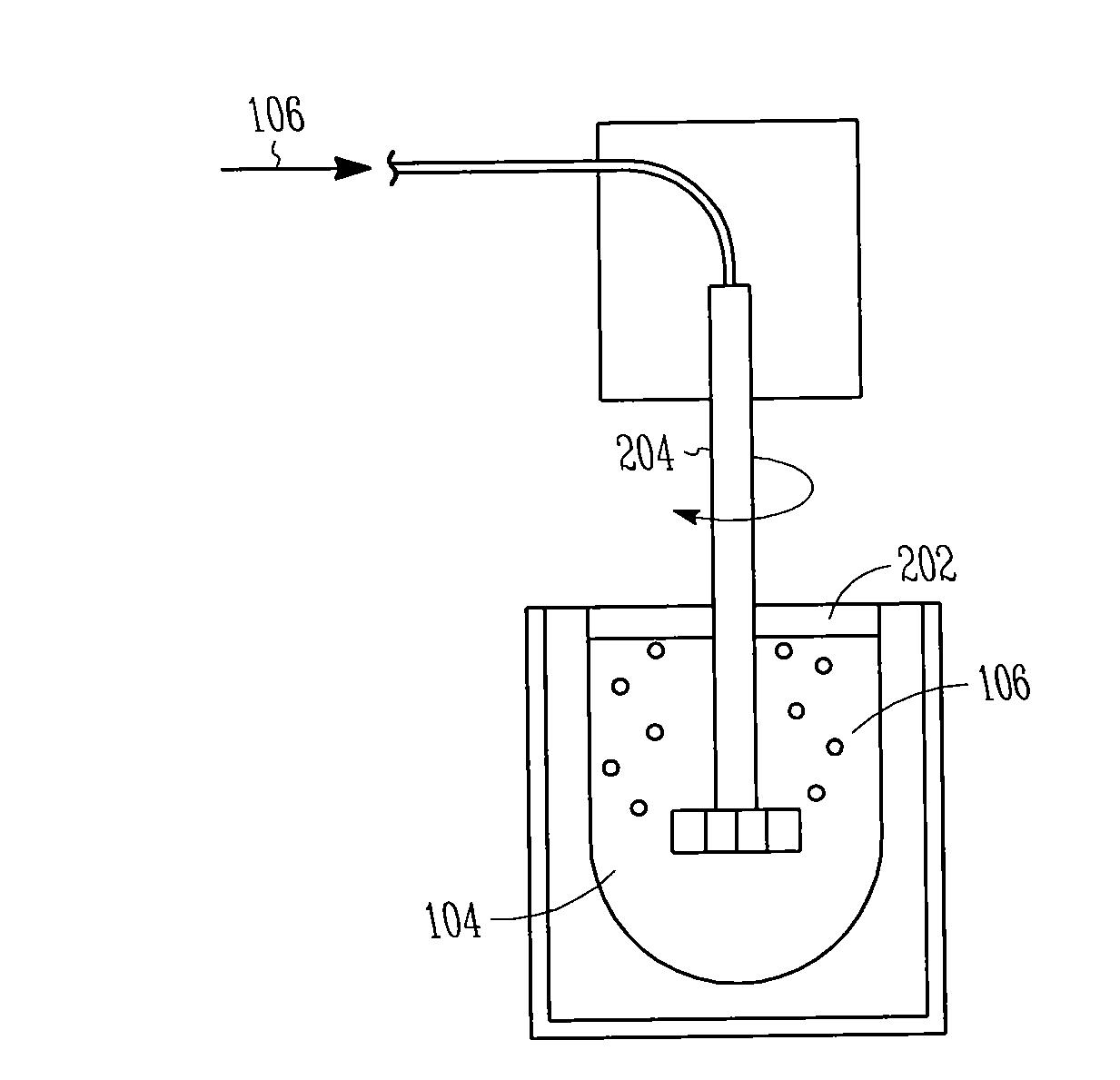
Patents
Literature
43results about "Aluminium halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium-containing composite oxide and its production method
ActiveUS20090148772A1Large volume capacity densityImprove securityCell electrodesLithium compoundsAlkaline earth metalDischarge rate
The present invention provides a lithium-containing composite oxide for a positive electrode for a lithium secondary battery, which has a large volume capacity density and high safety, and excellent durability for charge and discharge cycles and charge and discharge rate property, and its production method.The lithium-containing composite oxide is represented by the general formula LipNxMyOzFa (where N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, Sn, alkaline earth metal elements and transition metal elements other than Co, Mn and Ni, 0.9≦p≦1.2, 0.965≦x<2.00, 0<y≦0.035, 1.9≦z≦4.2, and 0≦a≦0.05), wherein when a powder of the lithium-containing composite oxide is classified into small particles with an average particle size of 2 μm≦Ds50≦8 μm and large particles with an average particle size of 10 μm≦Dl50≦25 μm, a content of the small particles is from 15 to 40% by weight and a content of the large particles is from 60 to 85% by weight, and 0.01≦ys≦0.06, 0≦yl≦0.02 and 0≦yl / ys<1, where (ys) is a ratio of the M element in the above general formula in the small particles and (yl) is a ratio of the M element in the general formula in the Large particles.
Owner:SUMITOMO CHEM CO LTD
Process for producing lithium-containing composite oxide for positive electrode for lithium secondary battery
ActiveUS20060154146A1Improve featuresSolve the small densityMagnesium halidesCell electrodesAlkaline earth metalNiobium
It is to provide a positive electrode active material for a lithium secondary battery, which has a large volume capacity density and high safety, is excellent in uniform coating properties and is excellent in the charge and discharge cyclic durability and low temperature characteristics even at a high charge voltage. A process for producing a lithium-containing composite oxide represented by the formula LipQqNxMyOzFa (wherein Q is at least one element selected from the group consisting of titanium, zirconium, niobium and tantalum, N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, alkaline earth metal elements and transition metal elements other than the Q element and the N element, 0.9≦p≦1.1, 0<q≦0.03, 0.97≦x<1.00, 0≦y<0.03, 1.9≦z≦2.1, q+x+y=1 and 0≦a≦0.02) from a lithium source, an Q element source and an N element source, and if necessary, at least one source selected from the group consisting of an M element source and a fluorine source, characterized by using as the Q element source an Q element compound aqueous solution having a pH of from 0.5 to 11.
Owner:SUMITOMO CHEM CO LTD
Carbon black
ActiveUS7300964B2Improve dynamic rigidityReduce hysteresisOrganic chemistryOther chemical processesOrganic groupOrganic compound
Carbon black having organic groups, the organic group containing a thiocyanate group. Also described is a process for the production of the carbon black, wherein carbon black is reacted with organic compounds containing a C—C double or triple bond, which is not part of an aromatic system, whose C—C double or triple bond is activated by at least one substituent, and the organic compound contains at least one thiocyanate group. The carbon black according to the invention can be used in rubber compounds.
Owner:UBS AG
Clean method for preparing layered double hydroxides
ActiveUS20080170978A1Efficient responseProtect environmentLithium compoundsManganese oxides/hydroxidesFiltrationCleaning methods
Disclosed is a clean method for preparing layered double hydroxides (LDHs), in which hydroxides of different metals are used as starting materials for production of LDHs by atom-economical reactions. The atom efficiency of the reaction is 100% in each case because all the atoms of the reactants are converted into the target product since only M2+(OH)2, M3+(OH)3, and CO2 or HnAn− are used, without any NaOH or other materials. Since there is no by-product, filtration or washing process is unnecessary. The consequent reduction in water consumption is also beneficial to the environment.
Owner:BEIJING UNIV OF CHEM TECH
Iodizing agent and process for preparation thereof
InactiveUS20060003024A1Avoid chemical degradationEasy to useBiocideGallium/indium/thallium compoundsIodised saltHydrotalcite
A method for preparation of iodizing agent for the use in the formulation of iodised salt that offers excellent stability of iodine in iodised salt is developed and the unrefined salt iodised with this compound was tested for its stability in presence of moisture, temperature and metal salts at higher temperature. The hydrotalcite type layered compound was used to prepare such compound and part of carbonate was substituted with iodate anion. The iodising agent exhibited excellent stability of iodine in iodised salt.
Owner:COUNCIL OF SCI & IND RES
Method and Apparatus for the Removal of Carbon Dioxide from a Gas Stream
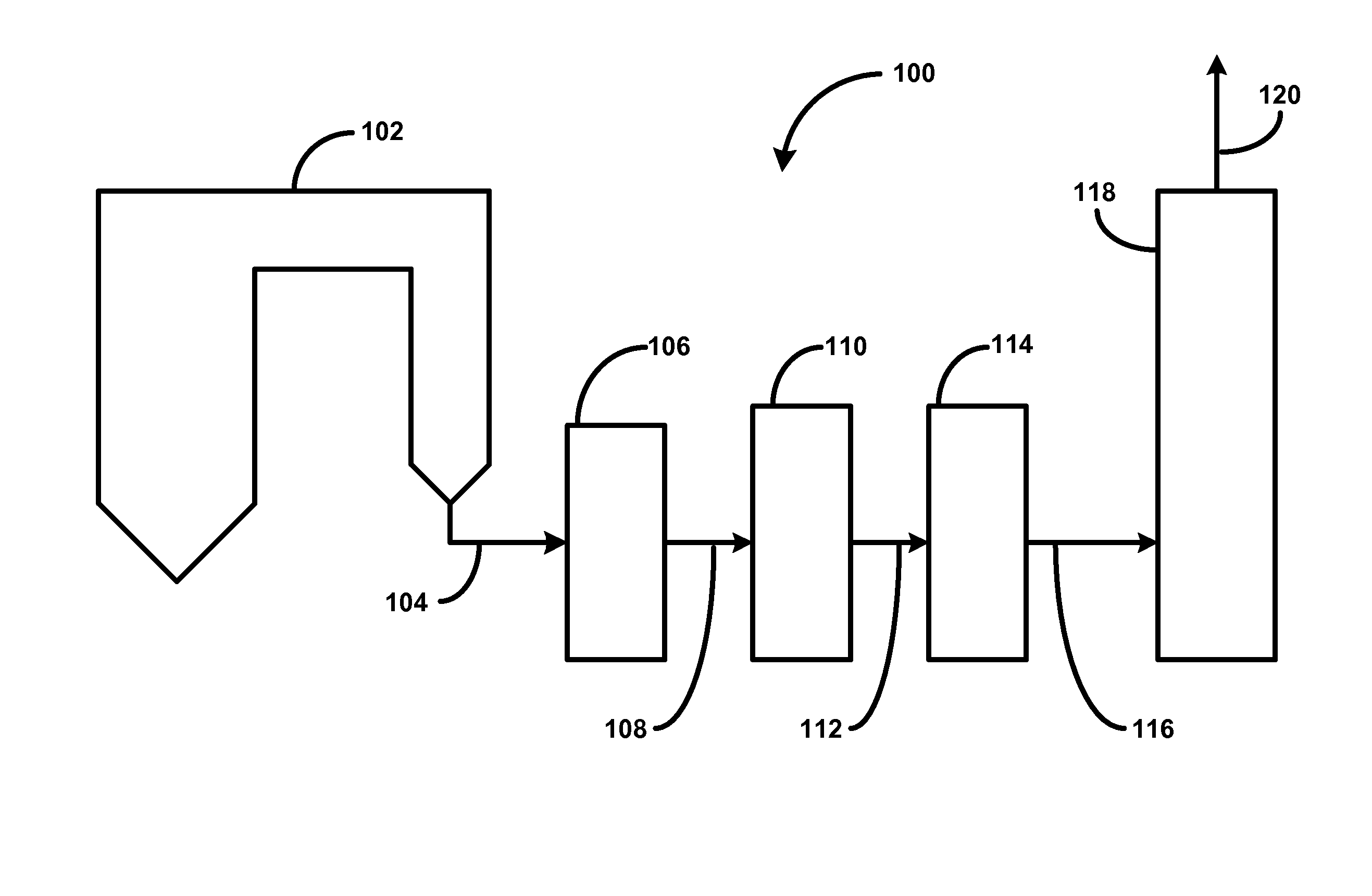
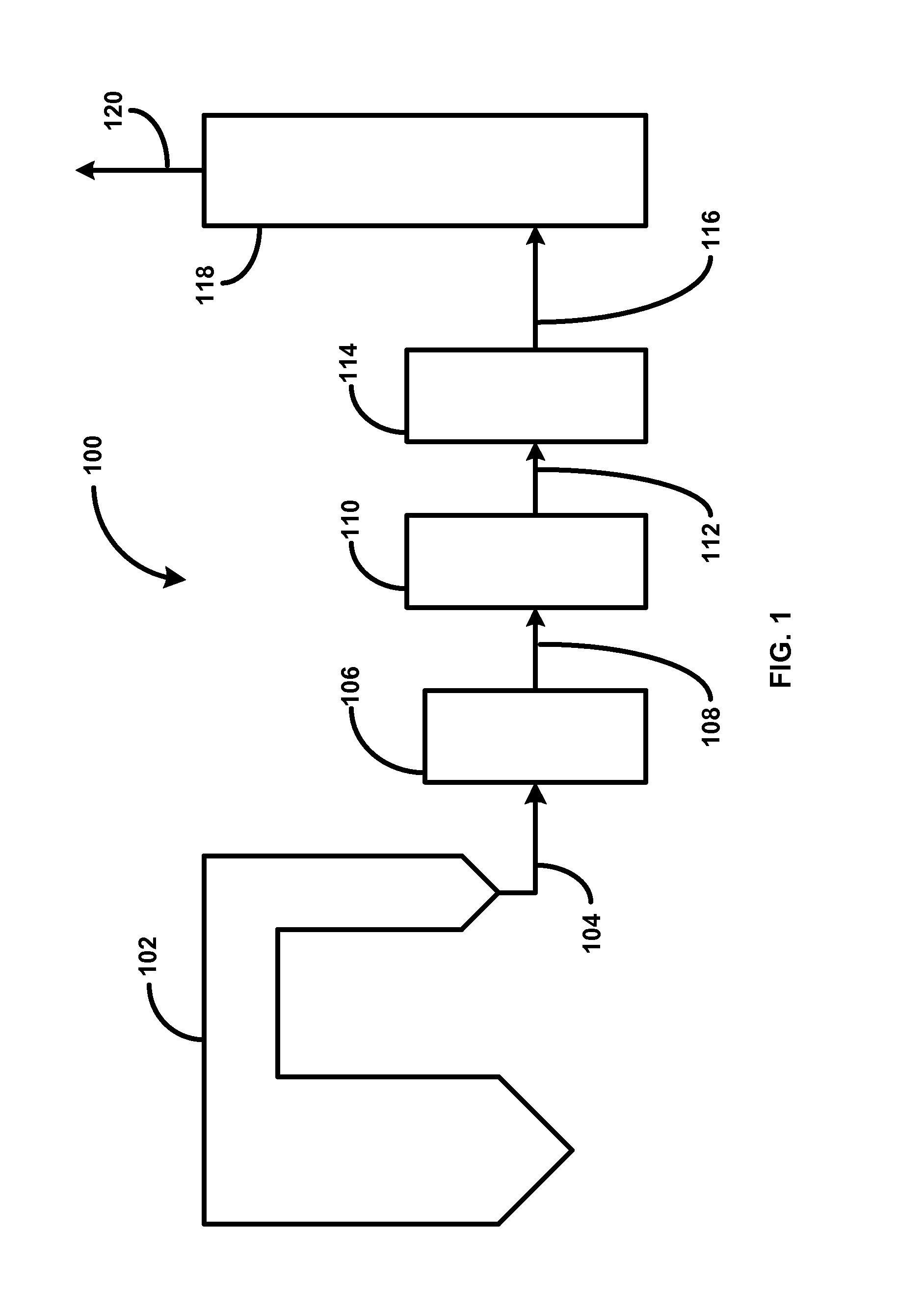
The invention provides methods and apparatuses for removing carbon dioxide from a gas stream. In particular, the invention provides methods and apparatuses for absorbing carbon dioxide from a coal-fired boiler flue gas stream using an absorbing solution and for regeneration of an alkaline component used in the absorbing solution. In one embodiment, the invention provides a method for removing carbon dioxide from a gas stream by contacting a gas stream containing carbon dioxide with an alkaline liquid stream; absorbing at least a portion of the carbon dioxide into the alkaline liquid stream to produce absorbed carbon dioxide; and catalyzing a reaction of the absorbed carbon dioxide to a form of carbonate. In other embodiments, the invention provides a method for producing salable calcium carbonate, calcium chloride, and carbon dioxide gas.
Owner:SINGH UDAY
Method for producing a halide brine
ActiveUS7087209B2Simple and inexpensive methodLow costMagnesium halidesGallium/indium/thallium compoundsHalogenSalt water
A method for producing halide brine wherein an alkali and a reducing agent are added to an aqueous fluid having a density greater than 8.30 lb / gal., (0.996 kg / L) water, waste water or sea water for example. The resulting fluid is then contacted with a halogen to form a halide brine. The reaction occurs in a conventional reactor such as a mixing tank.
Owner:TETRA TECH INC
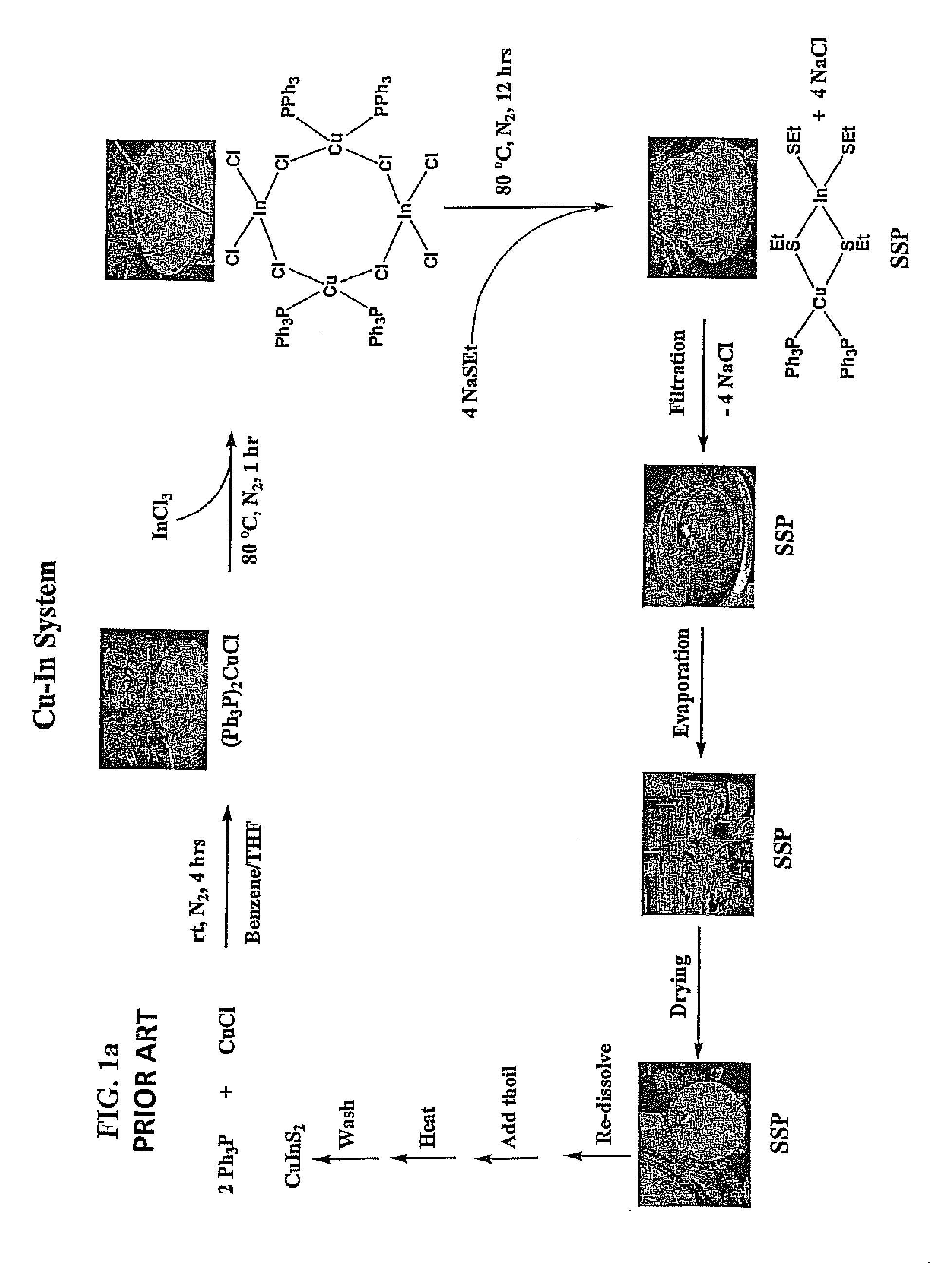
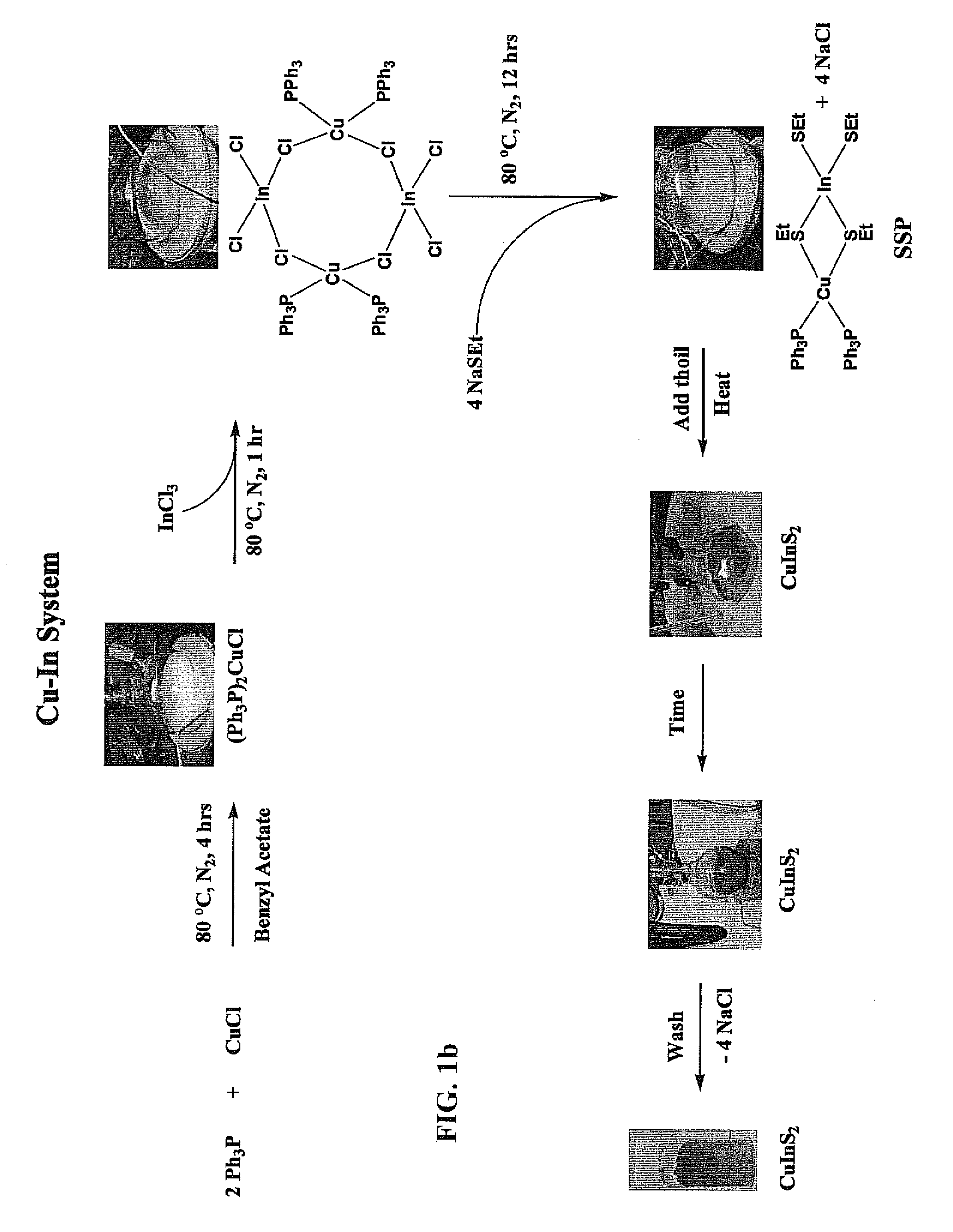
One-pot synthesis of chalcopyrite-based semi-conductor nanoparticles
ActiveUS8231848B1Material nanotechnologyPhosphorus sulfur/selenium/tellurium compoundsReaction temperatureSolvent
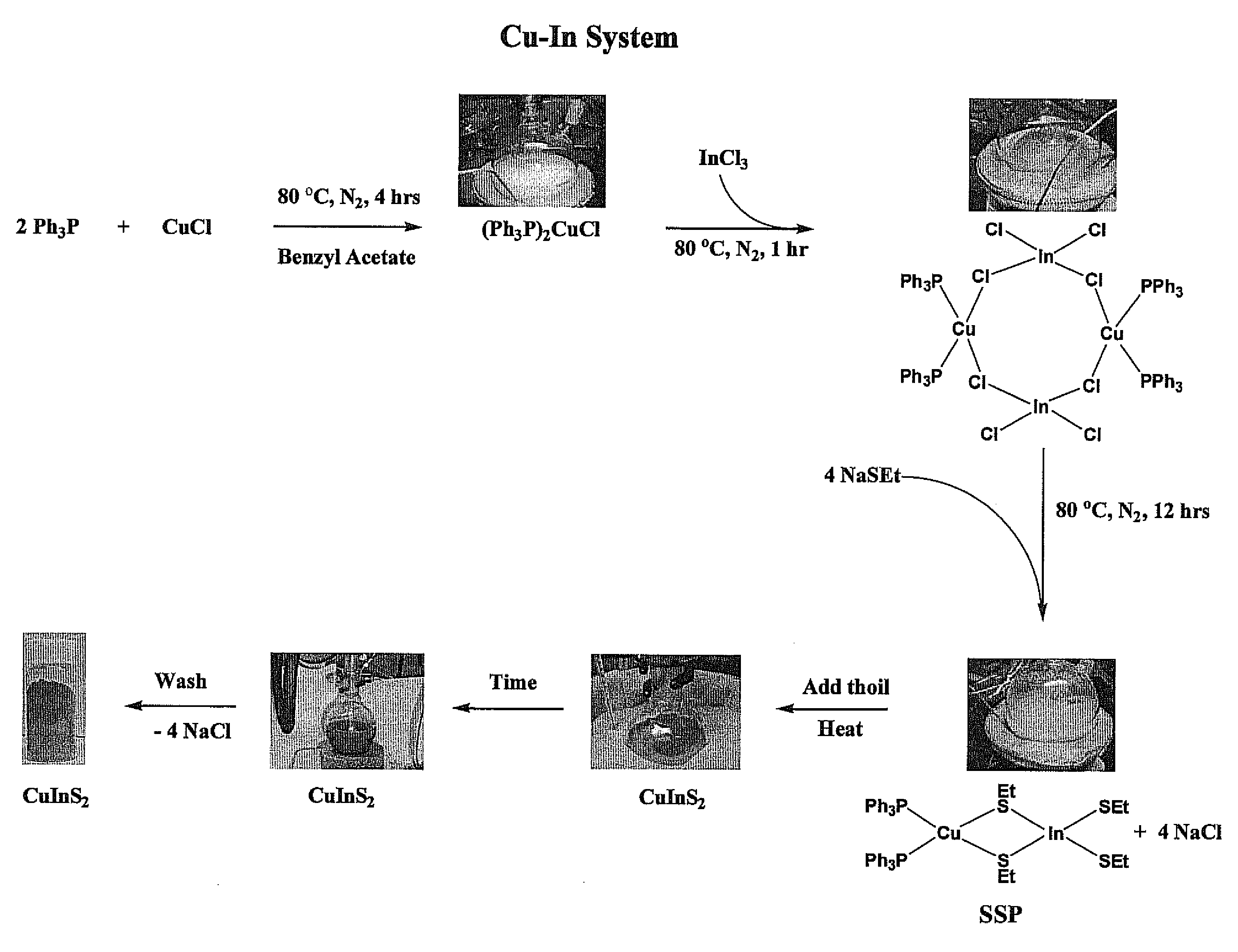
Ternary and quaternary Chalcopyrite CuInxGa1-xSySe2-y (CIGS, where 0≦x and y≦1) nanoparticles were synthesized from molecular single source precursors (SSPs) by a one-pot reaction in a high boiling solvent using salt(s) (i.e. NaCl as by-product) as heat transfer agent via conventional convective heating method. The nanoparticles sizes were 1.8 nm to 5.2 nm as reaction temperatures were varied from 150° C. to 190° C. with very high-yield. Tunable nanoparticle size is achieved through manipulation of reaction temperature, reaction time, and precursor concentrations. In addition, the method developed in this study was scalable to achieve ultra-large quantities production of tetragonal and quaternary Chalcopyrite CIGS nanoparticles.
Owner:ZHEJIANG SHANGYUE OPTOELECTRONICS TECH
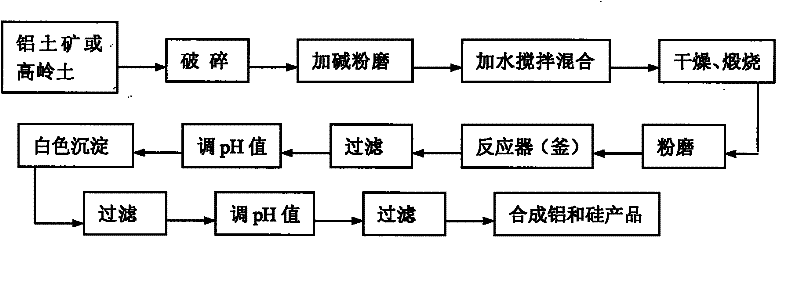
New process for producing aluminum and silicon chemical products by low-grade bauxite or kaolin raw material based on alkaline method
InactiveCN102198956AHigh whitenessGuaranteed whitenessSilicaAluminium halidesLower gradeChemical products
The invention belongs to the field of comprehensive utilization of a low-grade bauxite or kaolin (siallitic soil) raw material, and in particular relates to processes such as wet-method alkalinizing calcination, separation of aluminum and silicon compounds and the like. The process comprises the following steps: mixing the ground siallitic soil raw material with a proper amount of strong alkali and water, drying and performing calcined activation; grinding the calcined material, and then adding the ground material and a proper amount of water into a reaction tank (reaction kettle) so as to bedissolved into NaAlO2 and Na2SiO3 solutions as well as a small amount of solid at a proper temperature within a period of time; filtering, separating and adjusting the pH value so as to separate out Al(OH)3 and H2SiO3; and filtering and adjusting the pH value once again so as to separate out AlCl3 and H2SiO3, wherein, the AlCl3 is used for synthesizing aluminium polychloride and the H2SiO3 is used for producing white carbon black. The process provided by the invention has the beneficial effects that the dissolution efficiency of the raw material can be obviously improved, the whiteness and yield of the aluminium polychloride are increased, the high-value white carbon black can be produced, and the heat consumption is lowered, thus achieving great economical and environmental benefits.
Owner:JIAOZUO UNIV
Clean method for preparing layered double hydroxides
ActiveUS8088349B2Prevent materialAvoid prolonged useLithium compoundsManganese oxides/hydroxidesCleaning methodsLayered double hydroxides
Disclosed is a clean method for preparing layered double hydroxides (LDHs), in which hydroxides of different metals are used as starting materials for production of LDHs by atom-economical reactions. The atom efficiency of the reaction is 100% in each case because all the atoms of the reactants are converted into the target product since only M2+(OH)2, M3+(OH)3, and CO2 or HnAn− are used, without any NaOH or other materials. Since there is no by-product, filtration or washing process is unnecessary. The consequent reduction in water consumption is also beneficial to the environment.
Owner:BEIJING UNIV OF CHEM TECH
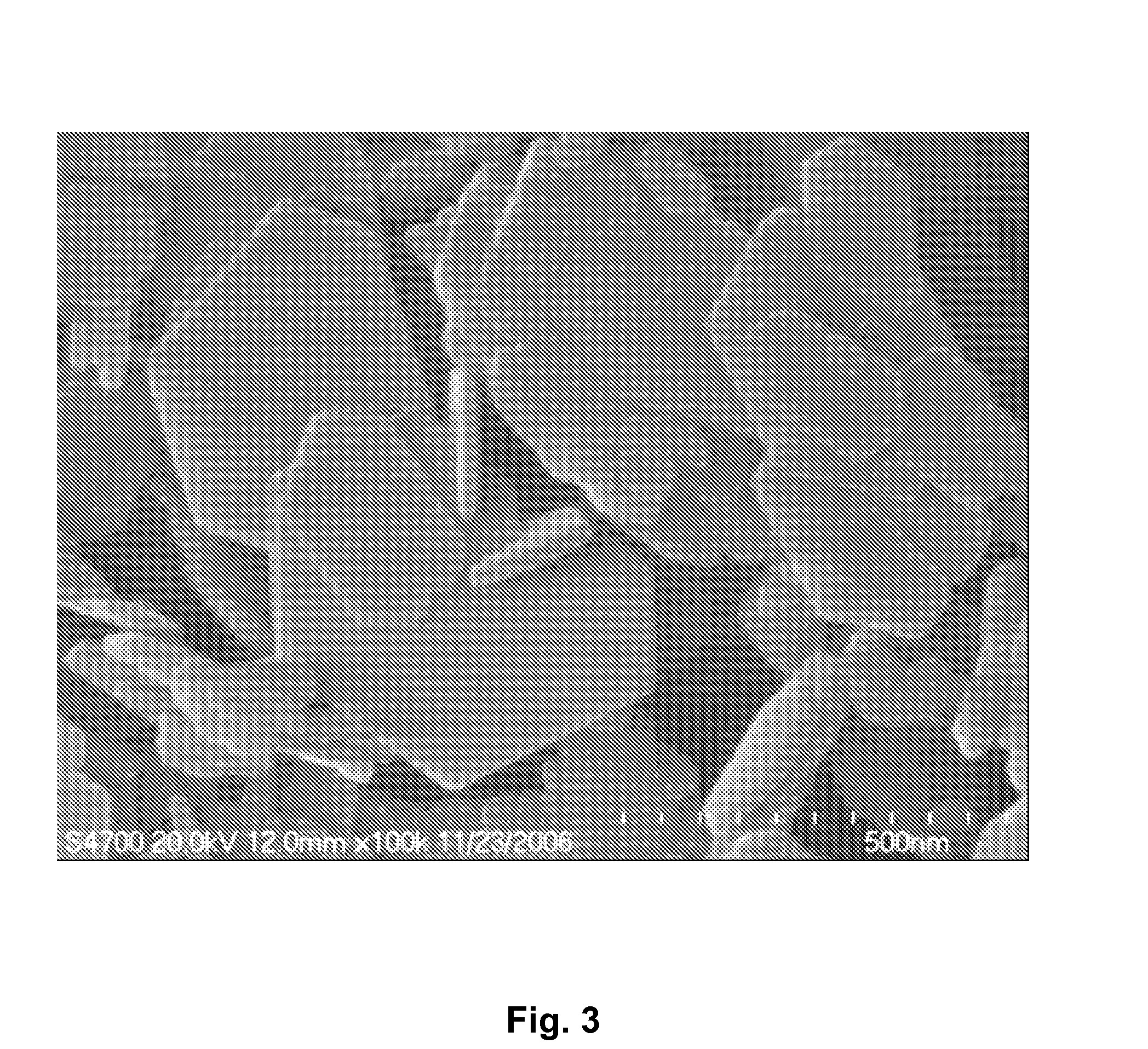
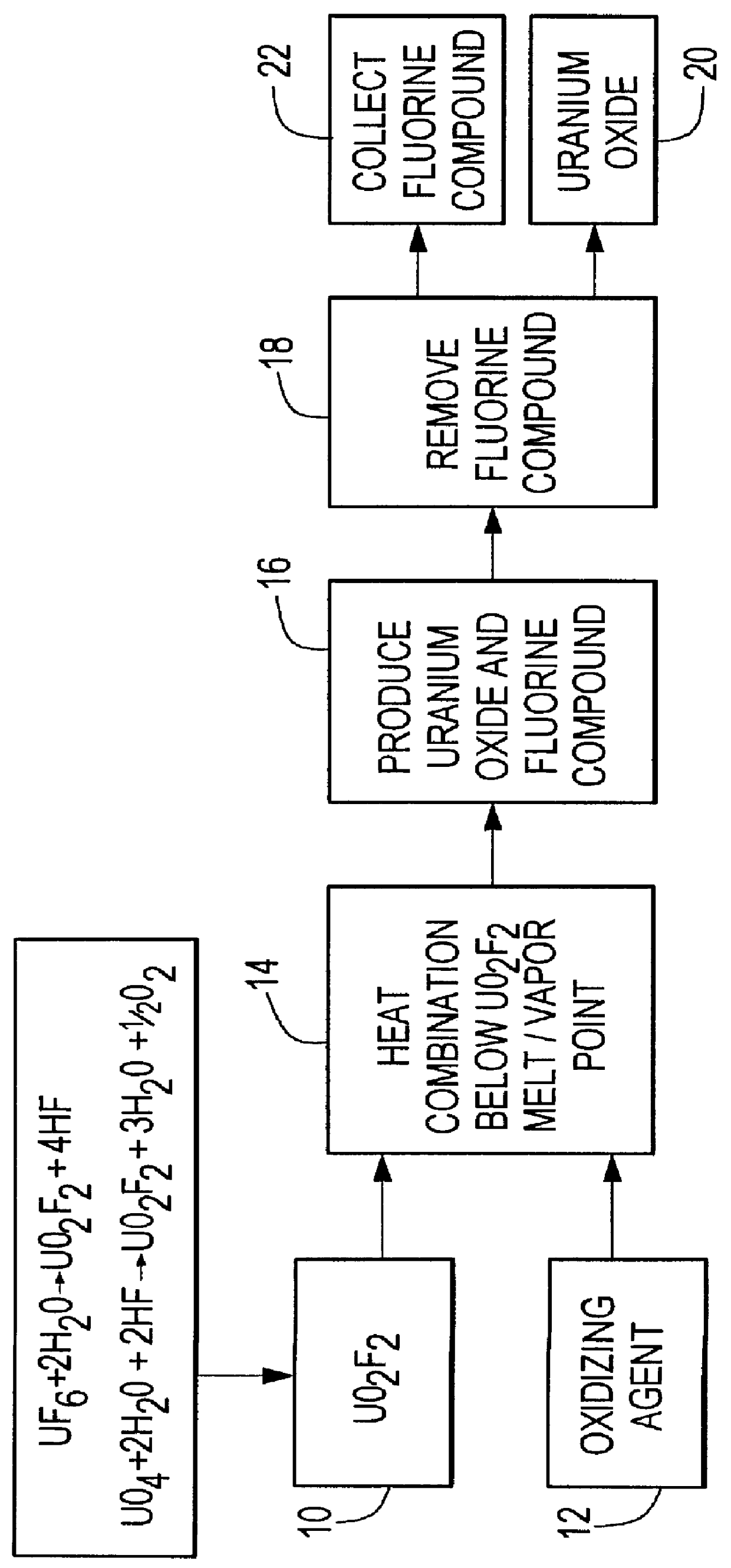
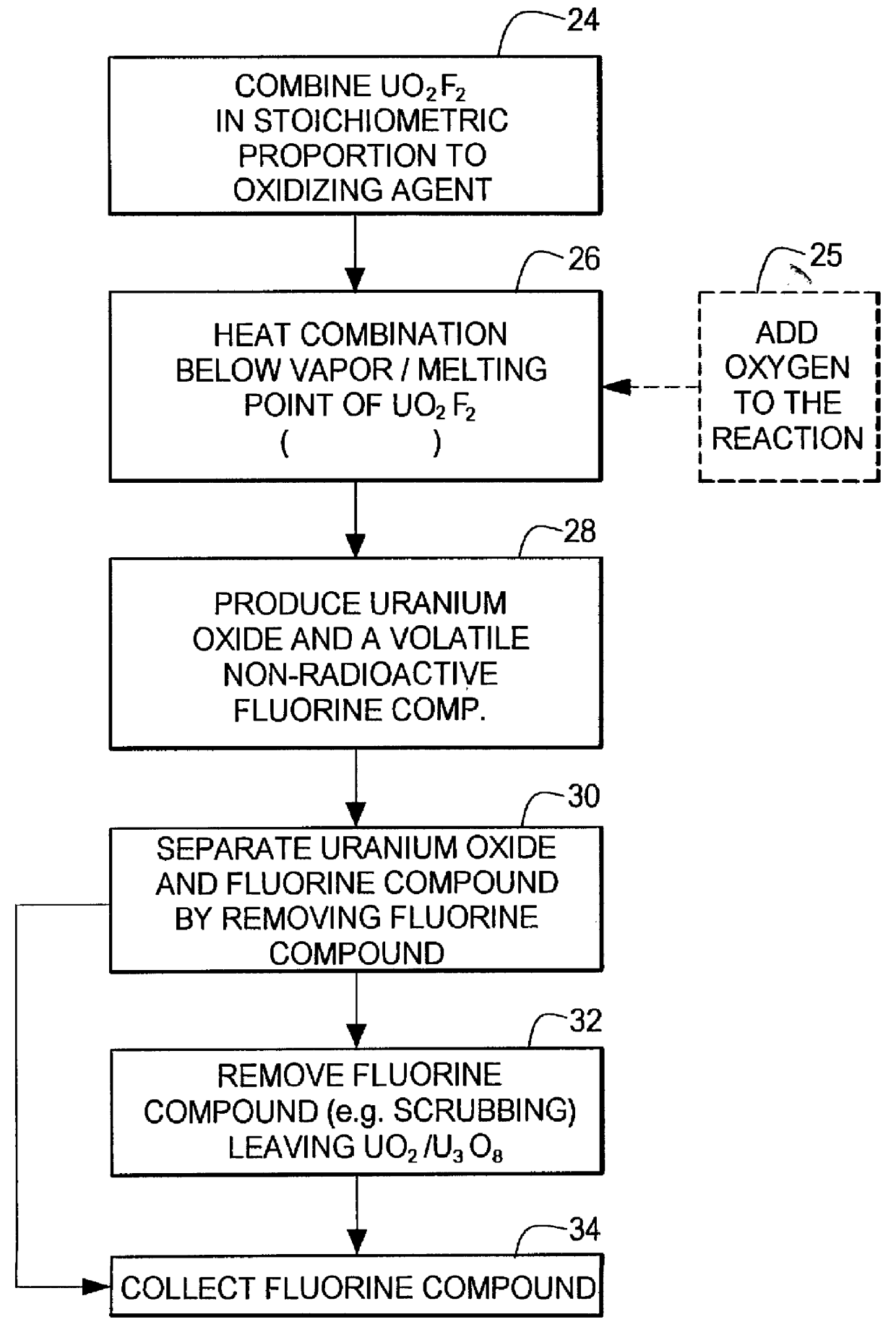
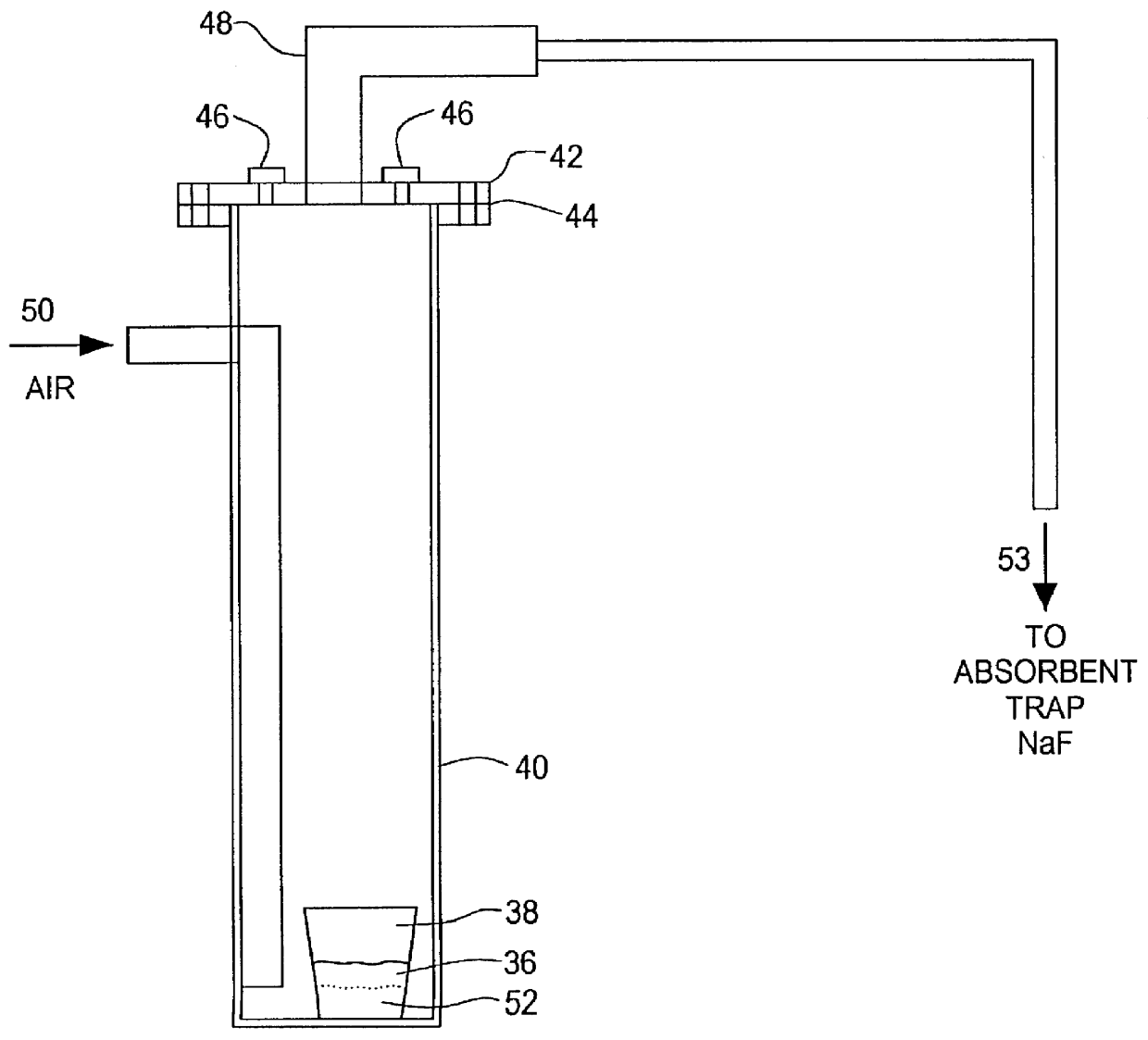
Method for producing uranium oxide from uranium oxyfluoride
InactiveUS6096281AReduced thermodynamic stabilityAvoid vaporizationPhosphorus halides/oxyhalidesFluoride preparationUranium oxideOxidizing agent
A method for producing uranium oxide includes combining uranium oxyfluoride and a solid oxidizing agent having a lower thermodynamic stability than the uranium oxide after "oxide"; heating the combination below the vapor point of the uranium oxyfluoride to sufficiently react the uranium oxyfluoride and the oxidizing agent to produce uranium oxide and a non-radioactive fluorine compound; and removing the fluorine compound after "compound".
Owner:INT ISOTOPES
Lithium-containing composite oxide and its production method
ActiveUS8192715B2Solve the small densityImprove securityCell electrodesLithium compoundsAlkaline earth metalVolumetric Mass Density
The present invention provides a lithium-containing composite oxide for a positive electrode for a lithium secondary battery, which has a large volume capacity density and high safety, and excellent durability for charge and discharge cycles and charge and discharge rate property, and its production method. The lithium-containing composite oxide is represented by the general formula LipNxMyOzFa (where N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, Sn, alkaline earth metal elements and transition metal elements other than Co, Mn and Ni, 0.9≦̸p≦̸1.2, 0.965≦̸x<2.00, 0<y≦̸0.035, 1.9≦̸z≦̸4.2, and 0≦̸a≦̸0.05), wherein when a powder of the lithium-containing composite oxide is classified into small particles with an average particle size of 2 μm≦̸Ds50≦̸8 μm and large particles with an average particle size of 10 μm≦̸Dl50≦̸25 μm, a content of the small particles is from 15 to 40% by weight and a content of the large particles is from 60 to 85% by weight, and 0.01≦̸ys≦̸0.06, 0≦̸yl≦̸0.02 and 0≦̸yl / ys<1, where (ys) is a ratio of the M element in the above general formula in the small particles and (yl) is a ratio of the M element in the general formula in the large particles.
Owner:SUMITOMO CHEM CO LTD
Carbon black
ActiveUS20050031528A1Reduce hysteresisHysteresis) is reducedOrganic chemistryOther chemical processesSimple Organic CompoundsOrganic group
Carbon black having organic groups, the organic group containing a thiocyanate group. Also described is a process for the production of the carbon black, wherein carbon black is reacted with organic compounds containing a C—C double or triple bond, which is not part of an aromatic system, whose C—C double or triple bond is activated by at least one substituent, and the organic compound contains at least one thiocyanate group. The carbon black according to the invention can be used in rubber compounds.
Owner:UBS AG
Method for purifying silicon
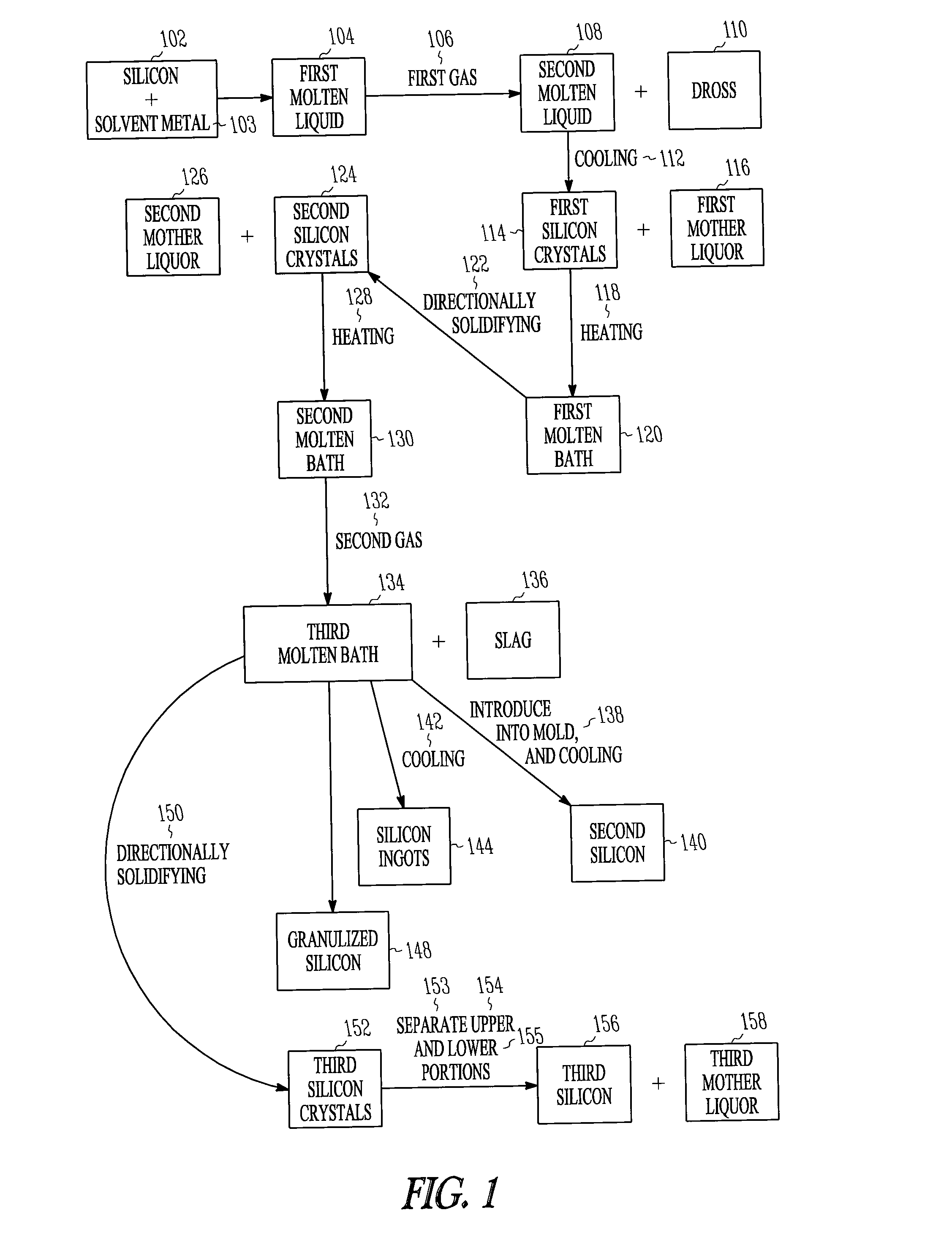
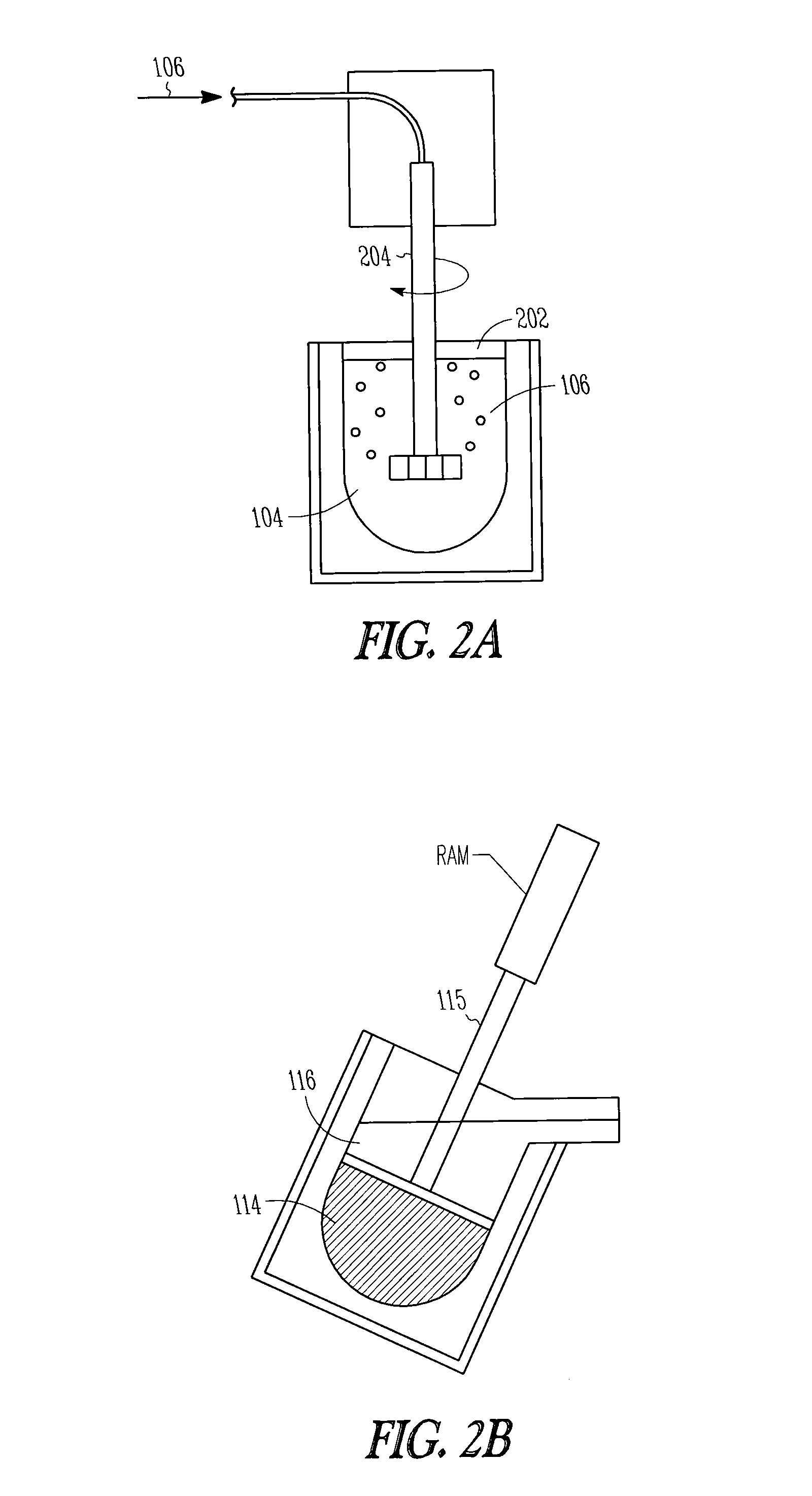
InactiveUS20100233064A1Efficiently provideBy zone-melting liquidsPretreated surfacesPhysical chemistryIngot
Owner:HIGHLAND MATERIALS INC
Processes for preparing chalcopyrite-type compounds and other inorganic compounds
InactiveUS7591990B2Low costEasy to preparePhosphorus sulfur/selenium/tellurium compoundsCell electrodesInorganic compoundElectrochemistry
Owner:TRANSFERT PLUS S E C
Manufacture of water chemistries
InactiveUS20080053104A1Effective and efficient and economically feasible processEfficient and effective processNitrogen compoundsElectrostatic separationPresent methodDisinfectant
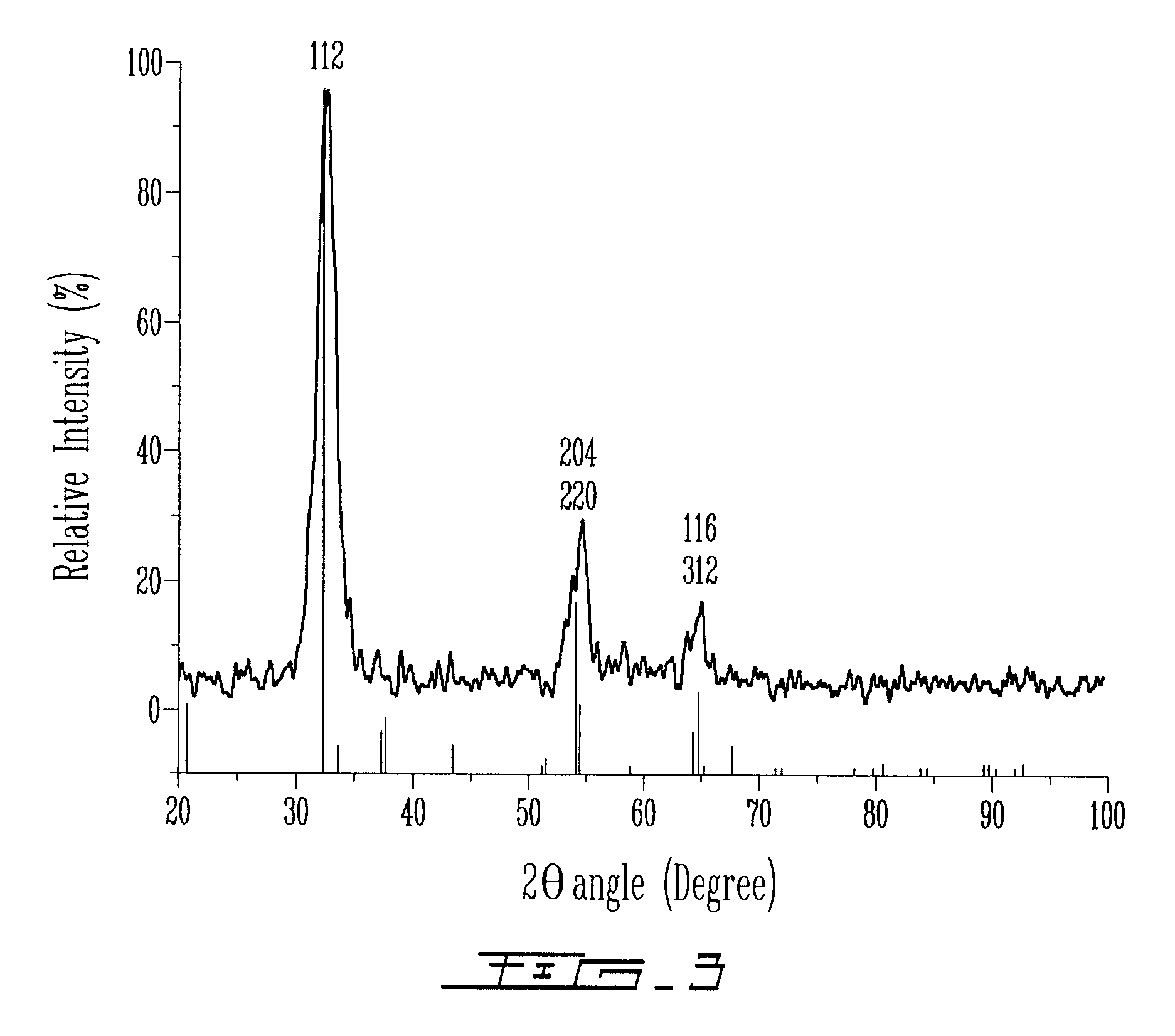
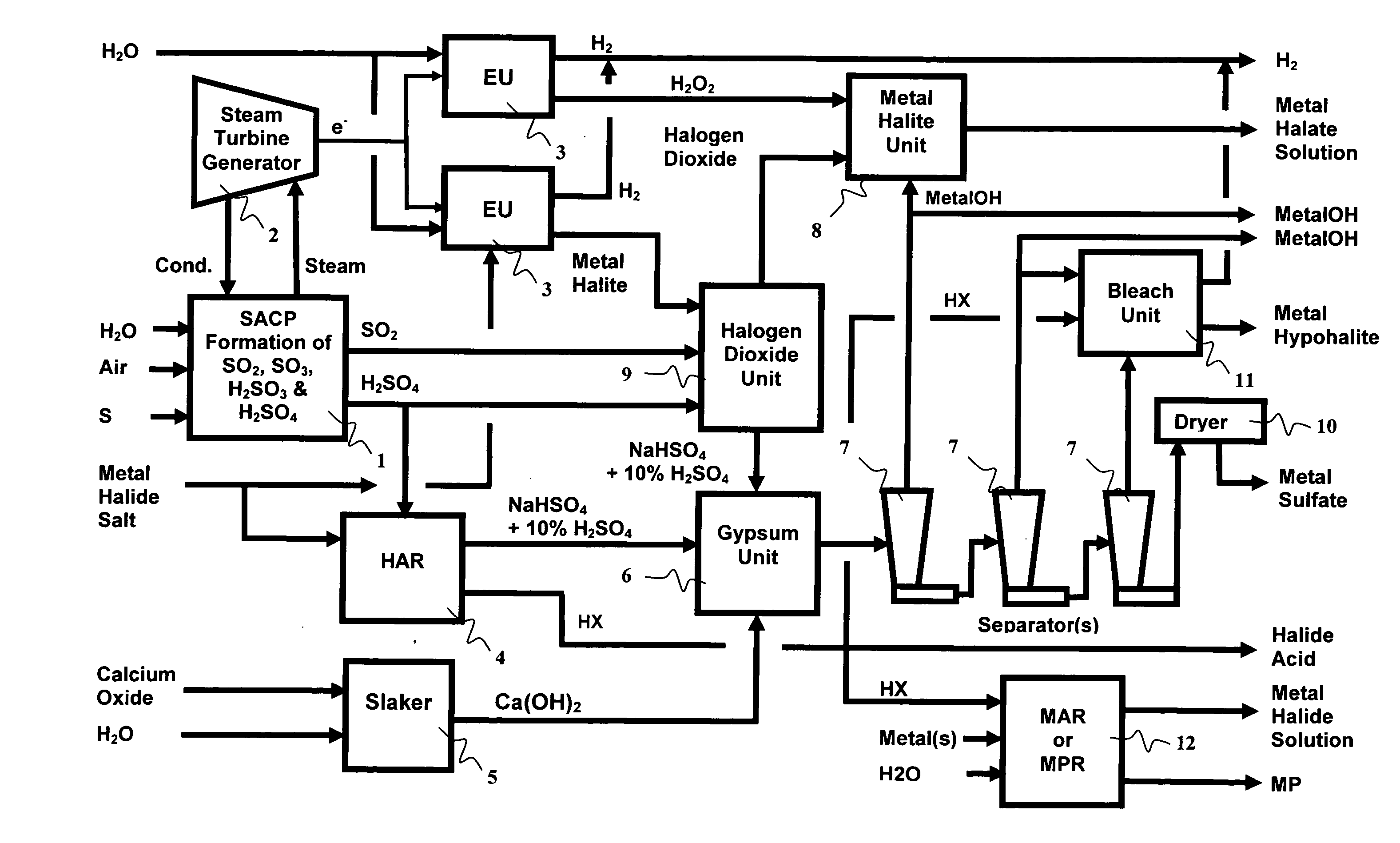
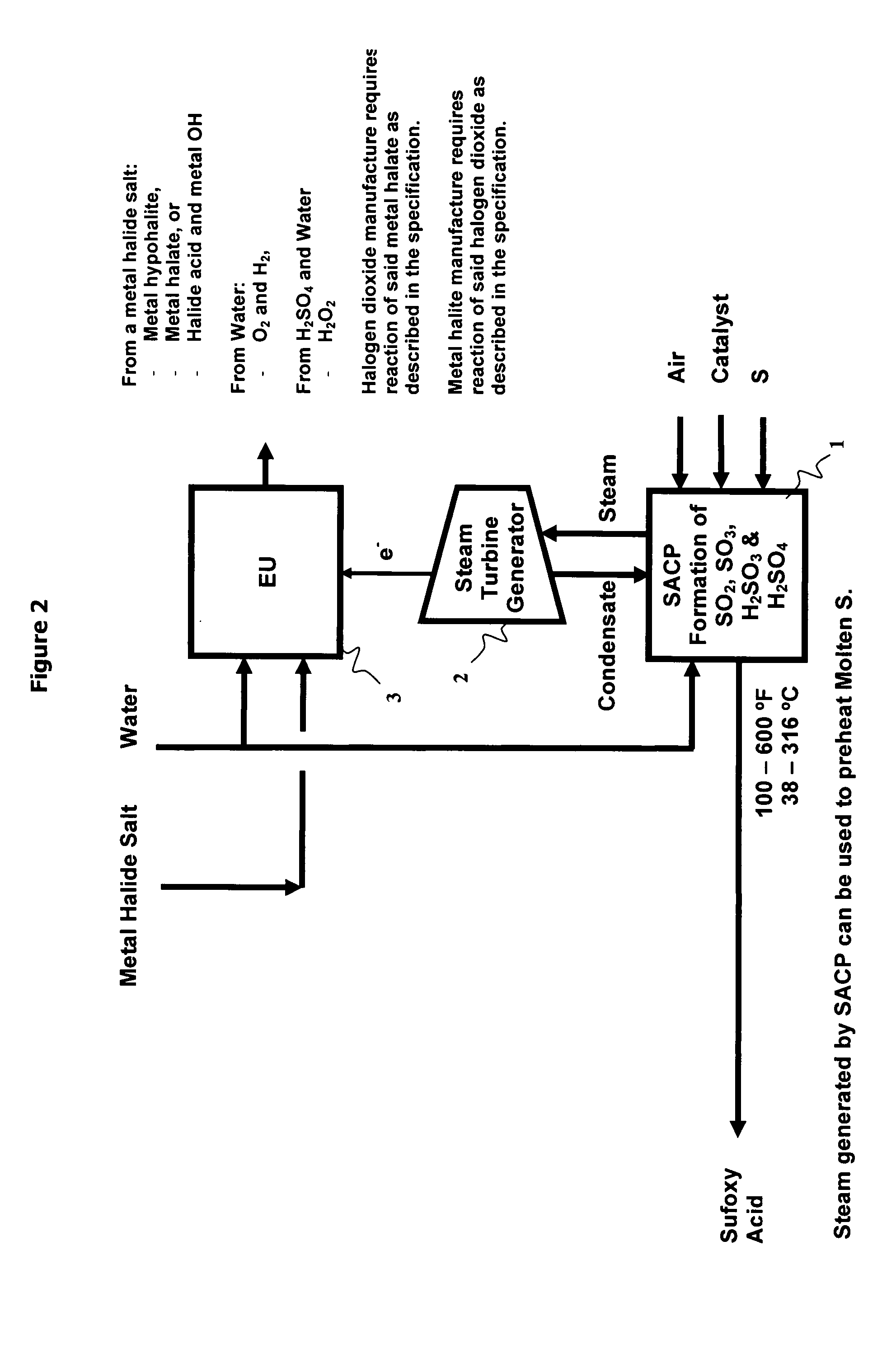
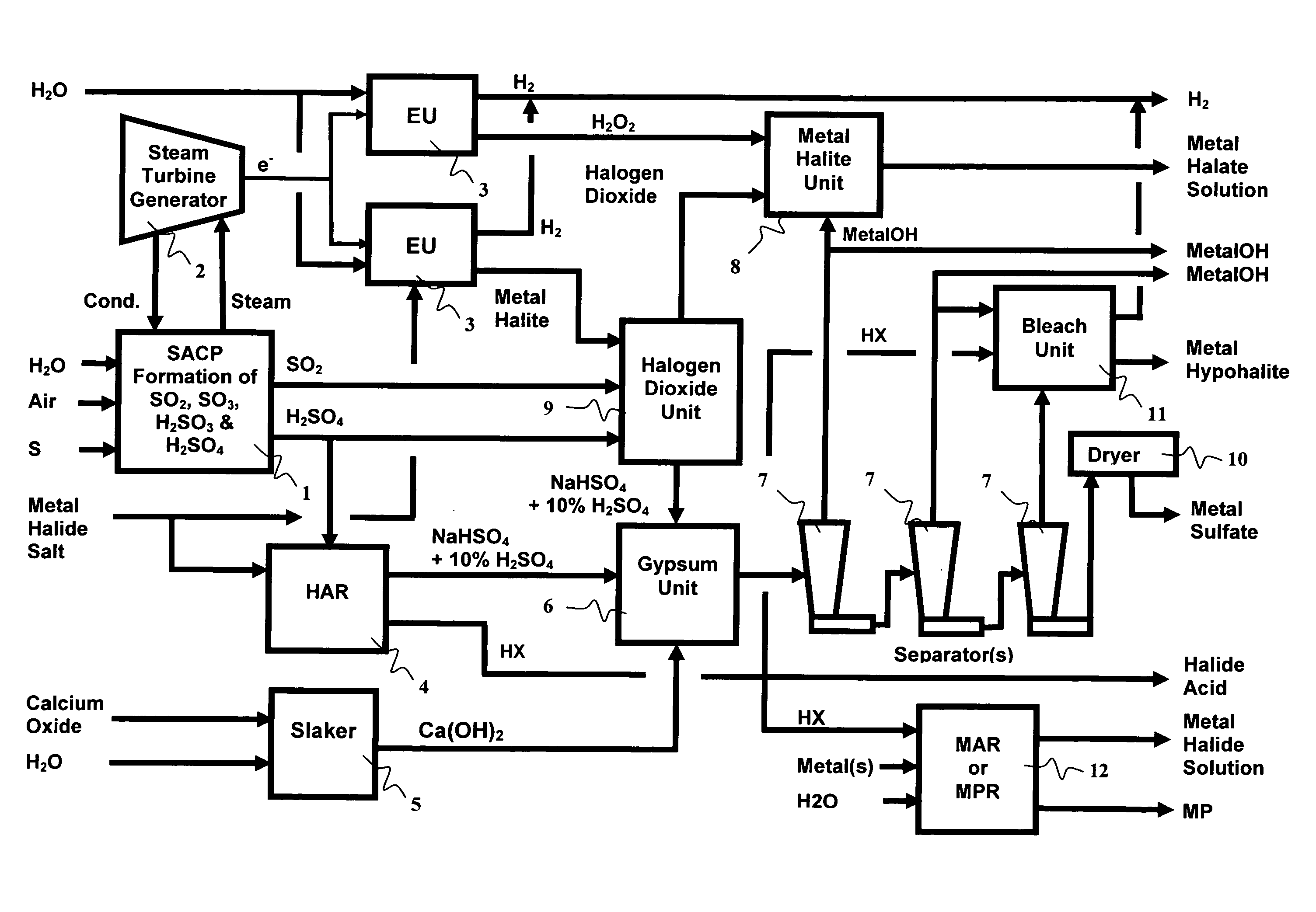
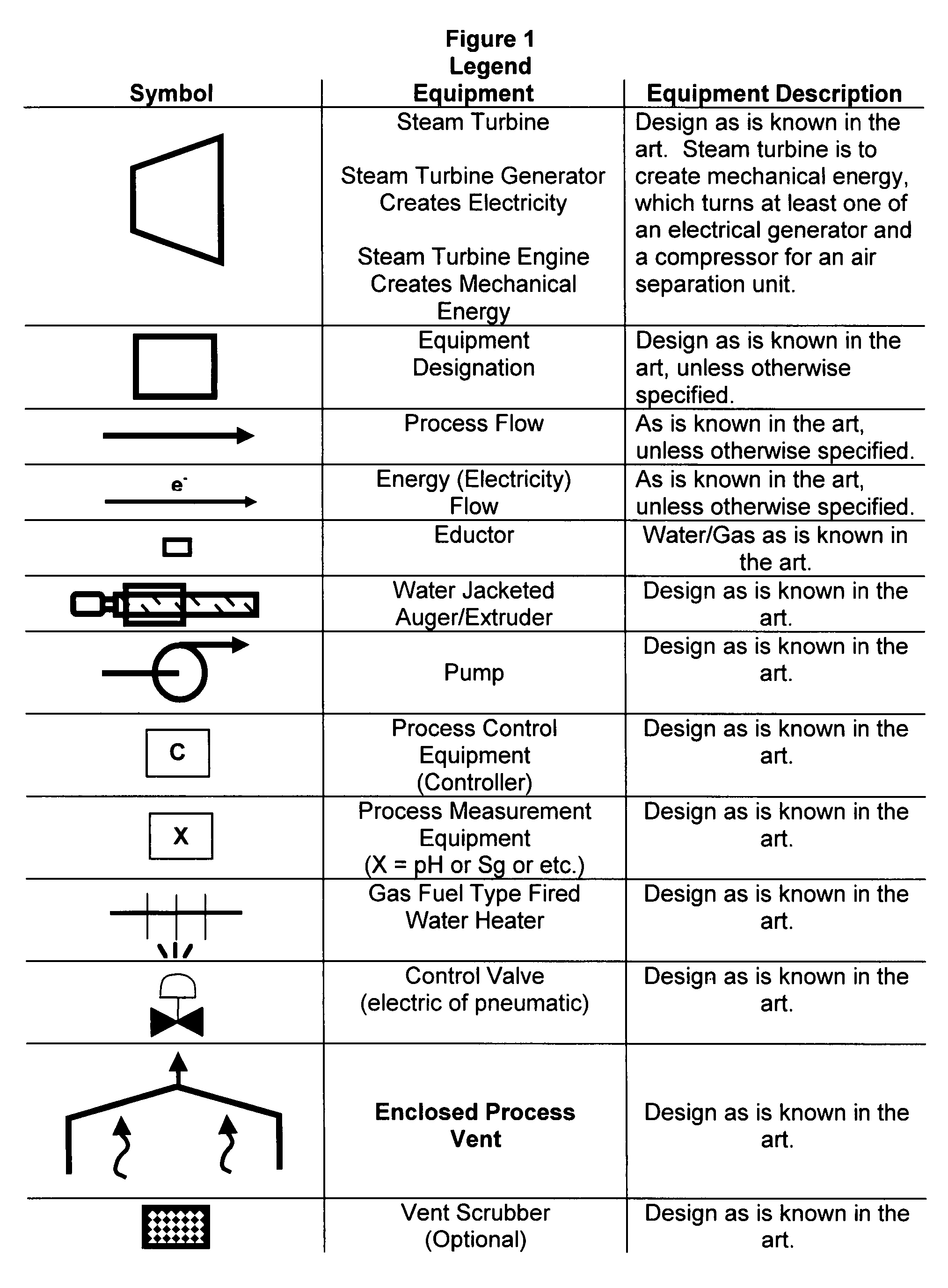
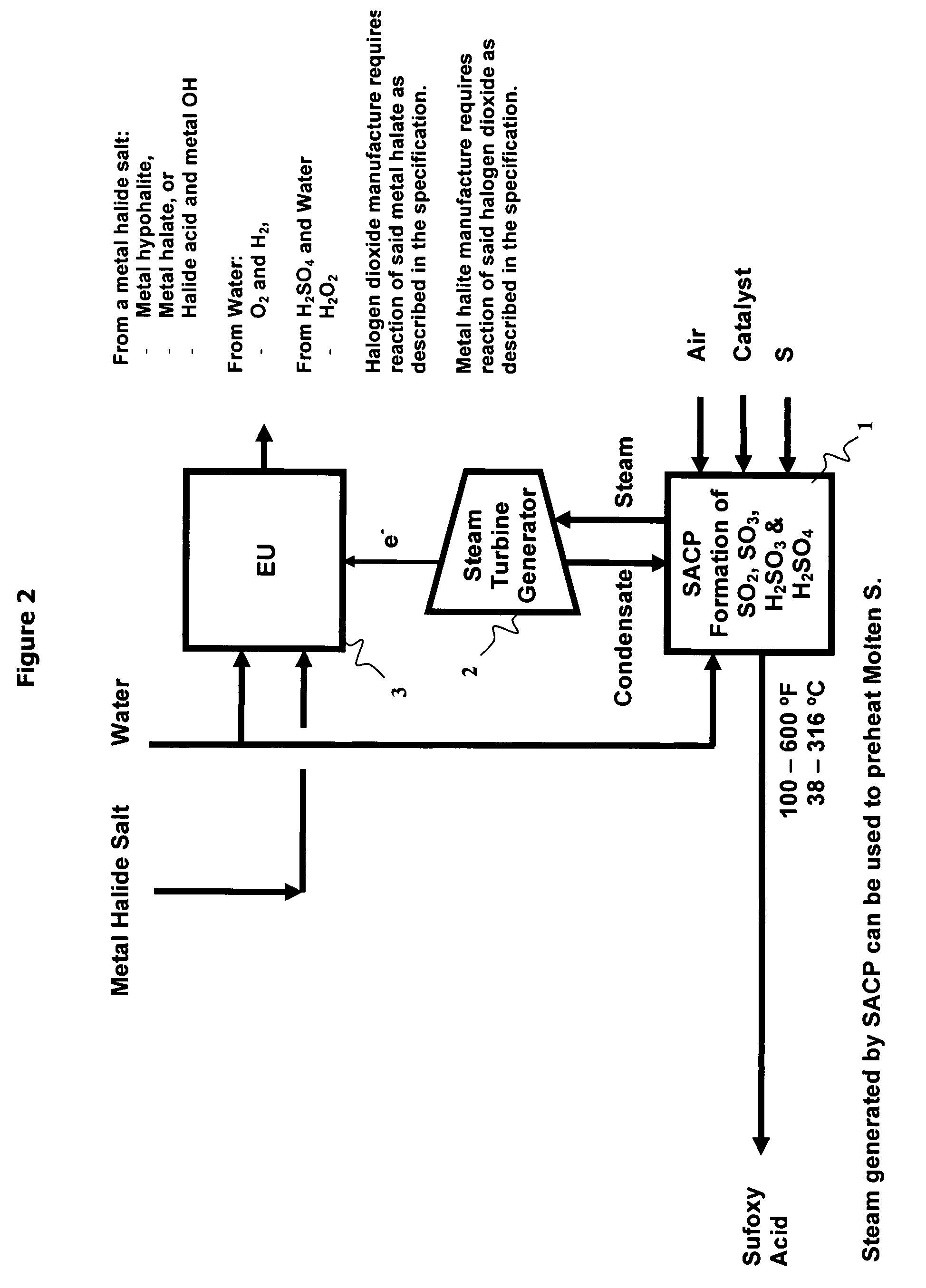
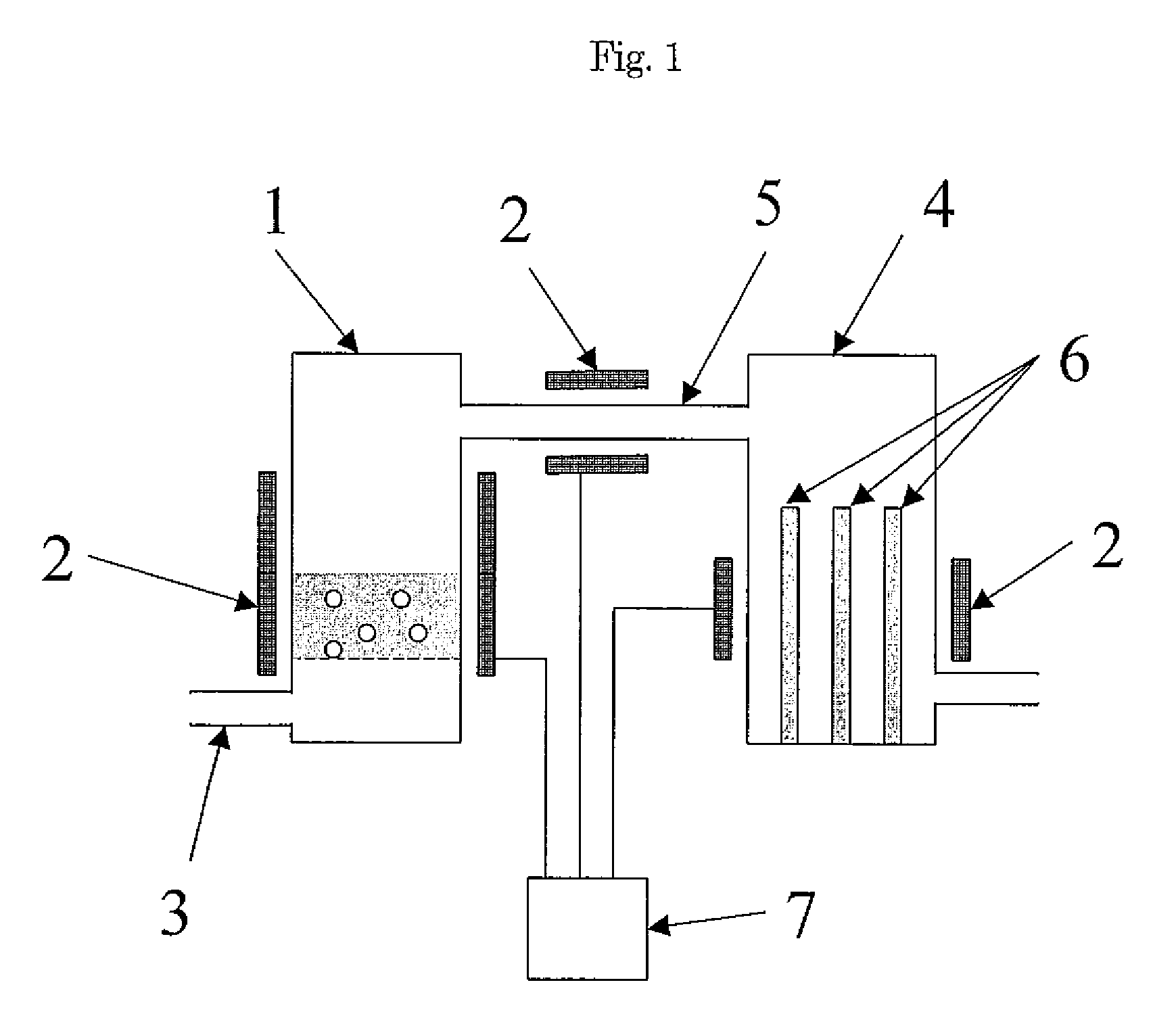
As population density increases, the transportation of hazardous chemicals, including acids and disinfectants, lead to an increased incidence of spills while the consequences of spills become more serious. While solutions of halide acids, hypohalites and halites are safer disinfectants for transportation, handling, storage and use than traditional gaseous chlorine, the manufacturing cost of these disinfectants has here-to-fore limited their use. Economical processes are presented for the manufacture of O2, halogen oxides, halide acids, hypohalites, and halates; as well as polynucleate metal compounds, metal hydroxides and calcium sulfate hydrate (gypsum). The instant invention presents methods and processes that incorporate the use of sulfur. This is while environmental regulators, such as the US EPA, require an increased removal of sulfur from hydrocarbon fuels, thereby creating an abundance of sulfur, such that the refining industry is in need of a way to dispose of said abundance of sulfur.
Owner:CLEARVALUE TECH
Processes for Preparing Chalcopyrite-Type Compounds and Other Inorganic Compounds
InactiveUS20080187483A1Low costEasy to preparePhosphorus sulfur/selenium/tellurium compoundsCell electrodesInorganic compoundElectrochemistry
There is provided a process for preparing compounds of formula M3M1A2. The process comprises reacting a compound of formula M2M1A2 with a compound of formula M3X2, in the presence of at least one coordinating solvent. M1 can be chosen from B3+, Al3+, Ga3+, In3+, Tl3+, Fe3+, and Au3+; M2 can be chosen from Li+, Na+, K+, Cs+, (T1)3Si—, and N(T2)4+; M3 can be chosen from Cu+, Ag+, Li+, Na+, K+, Cs+, Rb+, Fr+, Au+, and Hg+; A can be chosen from S and Se; and X2 can be chosen from Cl−, Br−, I−, F−, CH3COO−, NO3−, and CN−. Such compounds can be used for various purposes in the field of electrochemistry.
Owner:TRANSFERT PLUS S E C
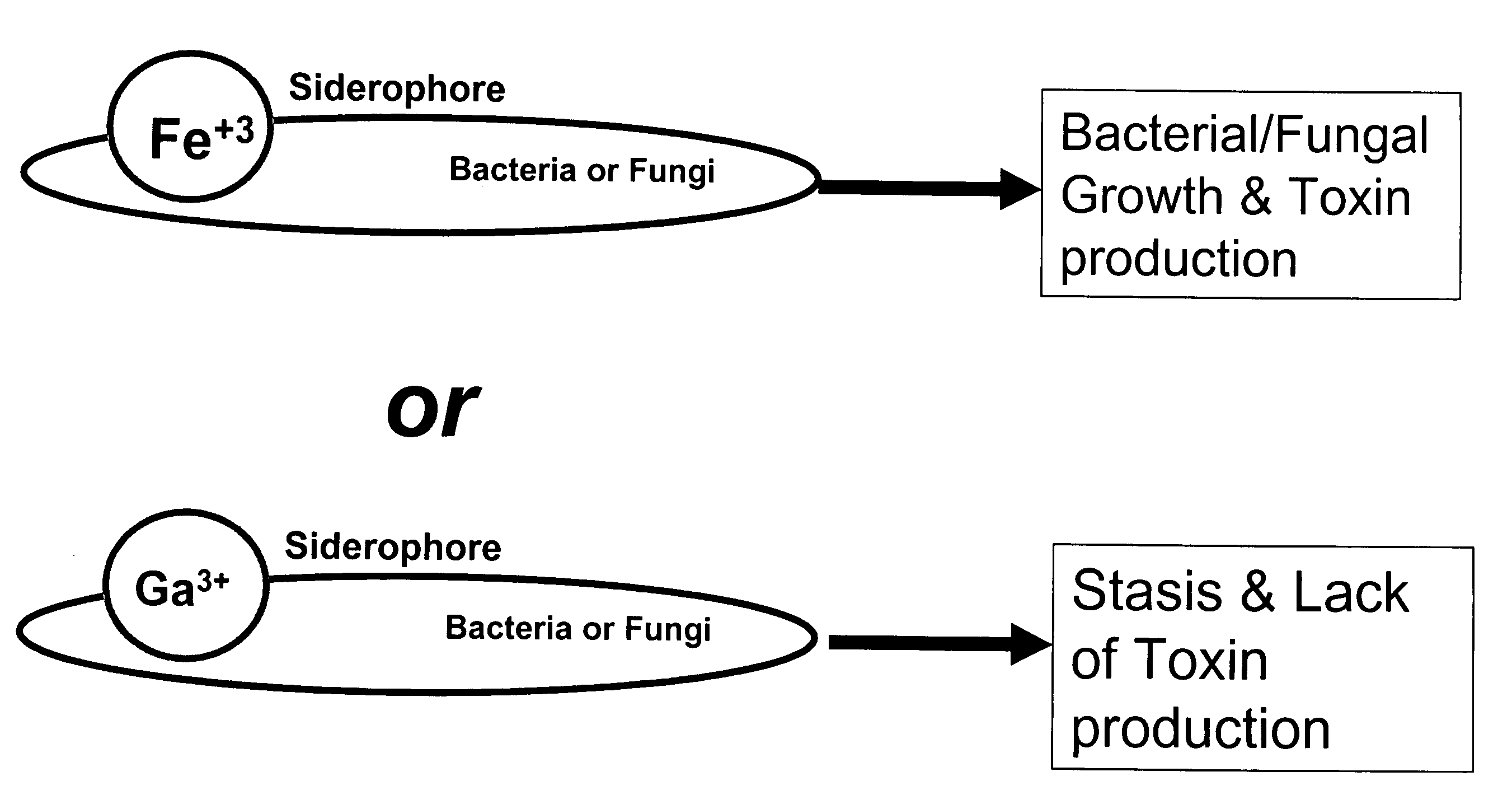
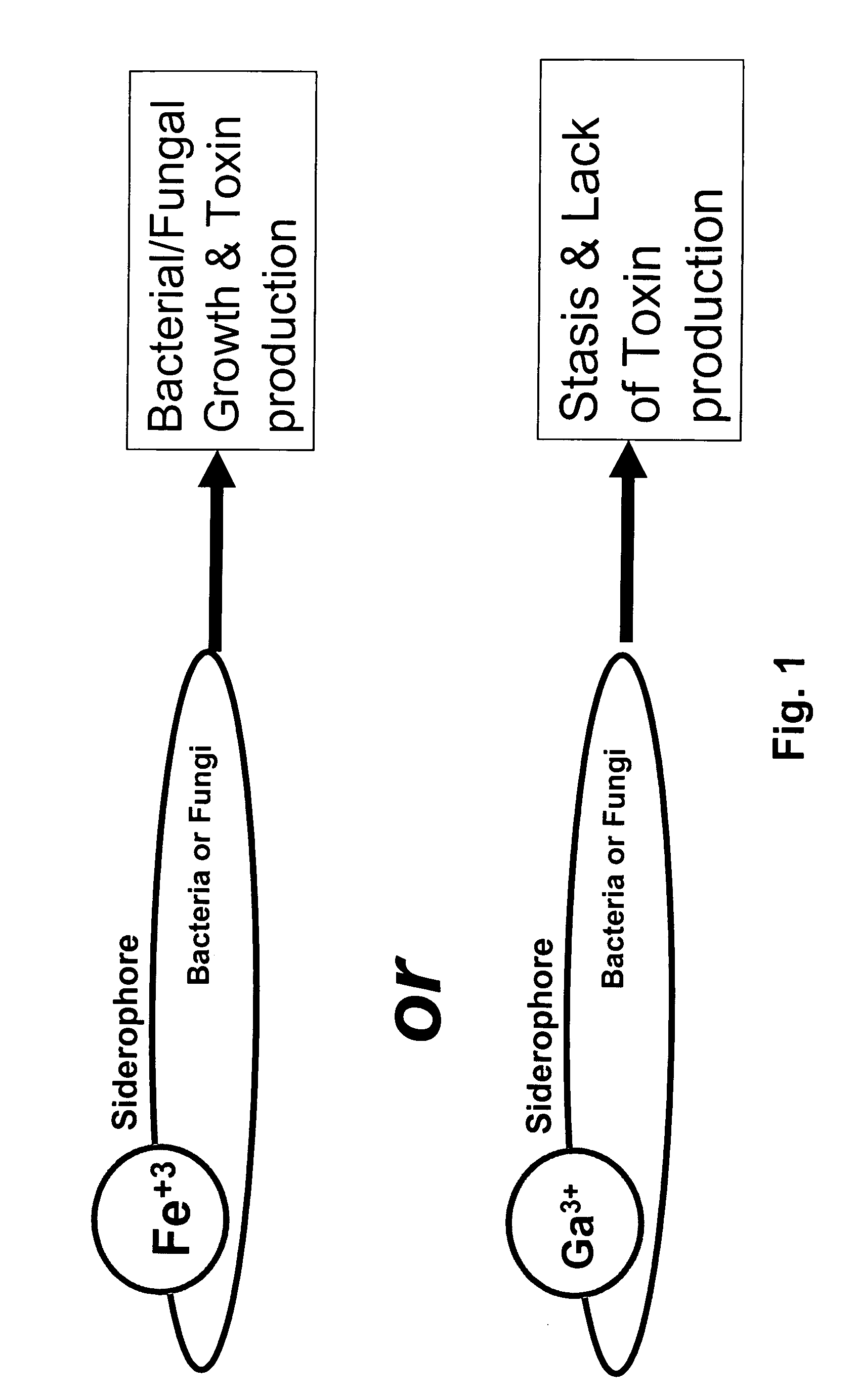
Growth control of oral and superficial microorganisms using gallium compounds
InactiveUS20110038809A1Easy to controlAntibacterial agentsOrganic active ingredientsDiseaseToothpaste
The present invention provides methods for treating or preventing diseases and disorders caused by iron-dependent pathogenic microorganisms, such as bacteria, fungi, and parasites, by applying a gallium compound to an affected area. In particular, the present invention provides methods for treating or preventing dental caries, vaginal infections, skin infections, and so forth. Gallium compounds can be formulated as toothpaste, mouthwash, cream, ointment, gel, solution, eye drops, suppository, and the like. Furthermore, the invention provides methods for controlling microbial growth on environmental surfaces, including those of toothbrush, denture, dental retainer, contact lens, catheter, food stuff, and so forth. In addition, the present invention provides animal feeds which contain gallium compounds that promote the animal growth and prevent the animals from infections as well as protect consumers from post processing infections.
Owner:MT SINAI SCHOOL OF MEDICINE
Removal of contaminants from by-product acids
InactiveUS7704470B2Effective treatmentProvide usageChlorine/hydrogen-chloride purificationSilicaPhosphate ionTitanium
Owner:HAYDOCK CONSULTING SERVICES LC
Method for producing polycrystalline silicon
InactiveUS20090136409A1High purityEfficient reductionSiliconFinal product manufactureMetal chlorideBoiling point
The present invention provides a method for producing polycrystalline silicon. The method for producing polycrystalline silicon comprises the steps of (A), (B), and (C),(A) reducing a chlorosilane represented by the formula (1) with a metal at a temperature Ti to obtain a silicon compound;SiHnCl4-n (1)wherein n is an integer of 0 to 3,(B) transferring the silicon compound to a zone having a temperature T2, wherein T1>T2; and(C) depositing polycrystalline silicon in the zone having a temperature T2, wherein the temperature T1 is not less than 1.29 times of a melting point (Kelvin unit) of the metal, and the temperature T2 is higher than a sublimation point or boiling point of the chloride of the metal.
Owner:SUMITOMO CHEM CO LTD
Iodizing agent and process for preparation thereof
InactiveUS7695707B2Reduce purification costsStable stateBiocideGallium/indium/thallium compoundsPhysical chemistryIodate salt
Owner:COUNCIL OF SCI & IND RES
High-efficiency synthetic method of nanometer aluminum particle
InactiveCN103058241ASmall sizeNarrow particle size distributionMaterial nanotechnologyAlkali-metal aluminates/aluminium-oxide/aluminium-hydroxide preparationAluminum IonRocket
The invention relates to a high-efficiency synthetic method of a nanometer aluminum particle. Products can be used for manufacturing thin film solar cell materials in order to improve the photoelectric conversion efficiency; the products can also be used for solid rocket fuel push agent. The invention belongs to the field of preparing novel functional materials. The nanometer aluminum particle regards a hyperbranched polymer as solvent, reducing agent and dispersing agent. After the hyperbranched polymer is contacted with trivalent aluminum ion, a spontaneous reduction reaction happens in the aqueous solution and a core-shell structure material is generated. An outer sphere of the core-shell structure material is an overrunning compound, and an inner sphere of the core-shell structure material is the nanometer aluminum particle. When a reaction system is placed in a strong magnetic field generator to be used for controlling the strength and the action time of the magnetic field, the reduction reaction efficiency and the product productivity can be improved by various degrees.
Owner:JIANGSU UNIV
Electrolyte based on aluminium iodide and its application
InactiveCN1911811AImprove conductivityRight melting pointLight-sensitive devicesIodide preparationSolventIonic liquid
The electrolyte based on AlI3 has the general expression of AlI3(L)n+mM+wI2+xG+yCP+zA, where, the ligand L is organic matter capable of coordinating with AlI3 and the coordination number n is 0-6; the solvent M may be amines, alcohols, nitriles or other organic solvent or ionic liquid and the coordination number m is 0-2000; w as the molar ratio between simple substance I and AlI3 is 0-0.5; the gel G is organic small molecular weight compound or organic high molecular weight compound with the molar ratio to AlI3 x of 0-1; the ceramic powder CP has molar ratio to AlI3 y of 0-5; and the additive A is compound for improving the performance of the electrolyte with the molar ratio to AlI3 z of 0-2. The electrolyte has high long term stability, high conductivity and proper smelting point, and may be used in dye sensitized solar cell and other energy converting and storing devices.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
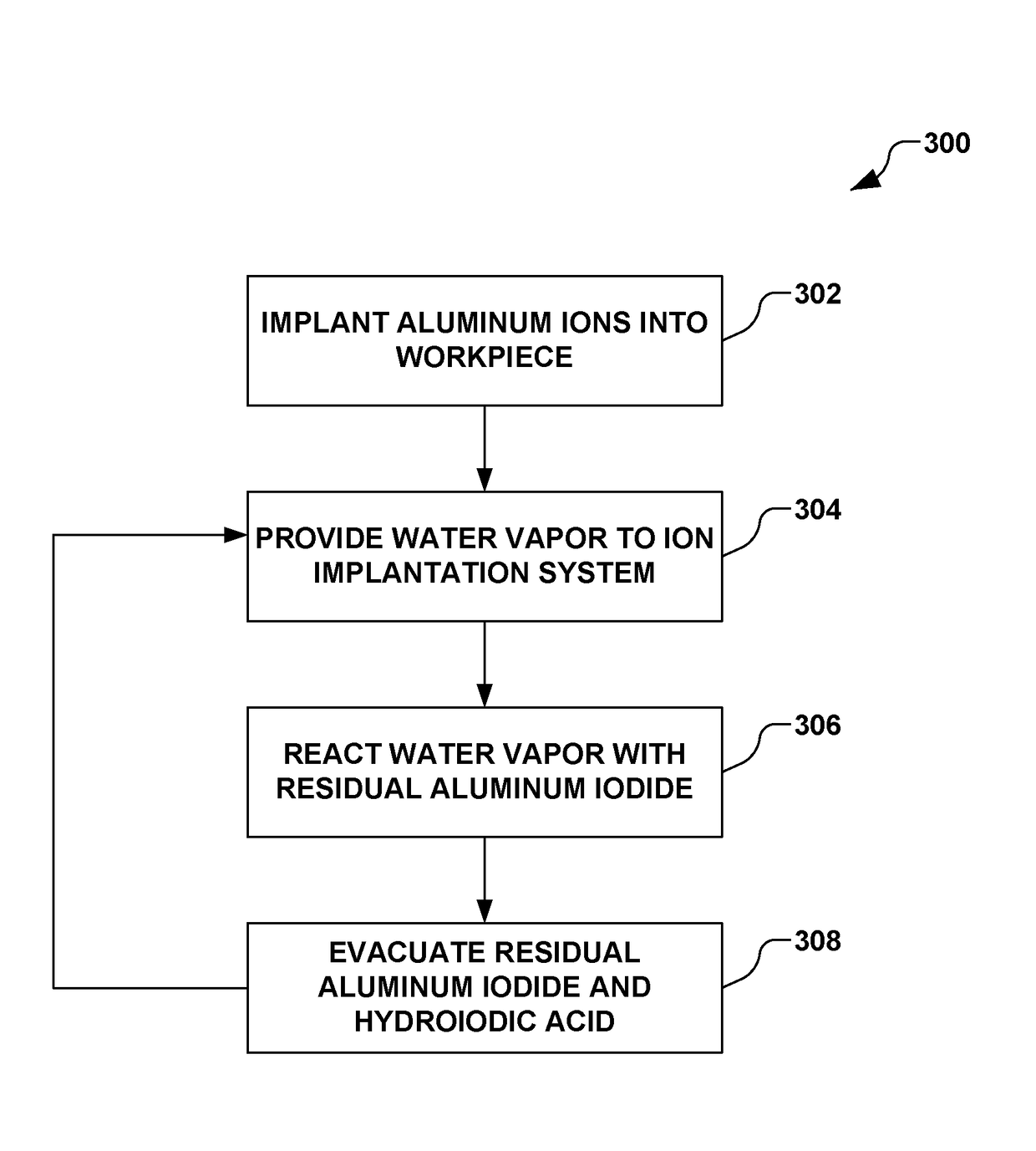
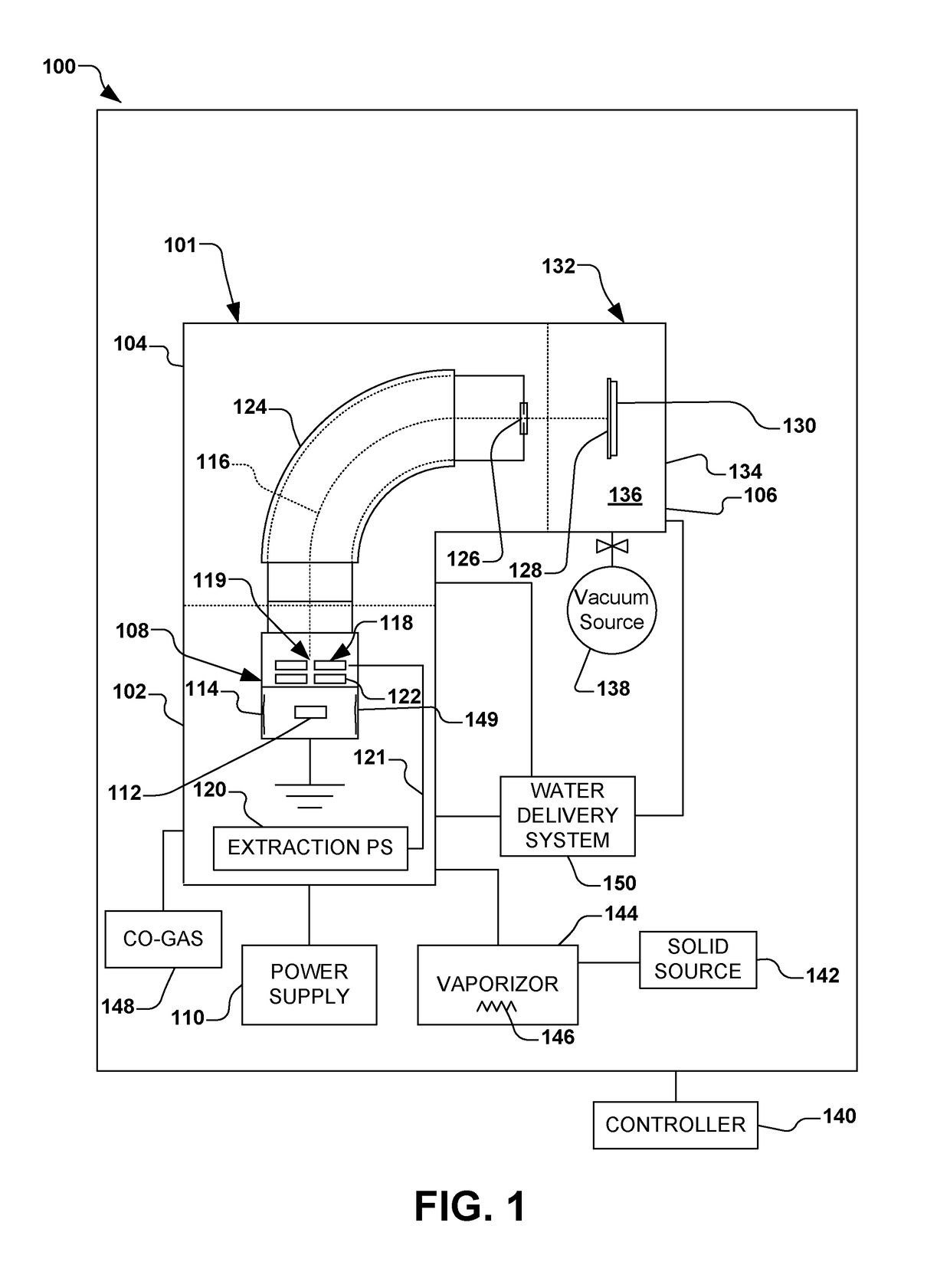
Implantation using solid aluminum iodide (AlI3) for producing atomic aluminum ions and in situ cleaning of aluminum iodide and associated by-products
An ion implantation system is provided having an ion source configured to form an ion beam from aluminum iodide. A beamline assembly selectively transports the ion beam to an end station configured to accept the ion beam for implantation of aluminum ions into a workpiece. The ion source has a solid-state material source having aluminum iodide in a solid form. A solid source vaporizer vaporizes the aluminum iodide, defining gaseous aluminum iodide. An arc chamber forms a plasma from the gaseous aluminum iodide, where arc current from a power supply is configured to dissociate aluminum ions from the aluminum iodide. One or more extraction electrodes extract the ion beam from the arc chamber. A water vapor source further introduces water to react residual aluminum iodide to form hydroiodic acid, where the residual aluminum iodide and hydroiodic acid is evacuated from the system.
Owner:AXCELIS TECHNOLOGIES
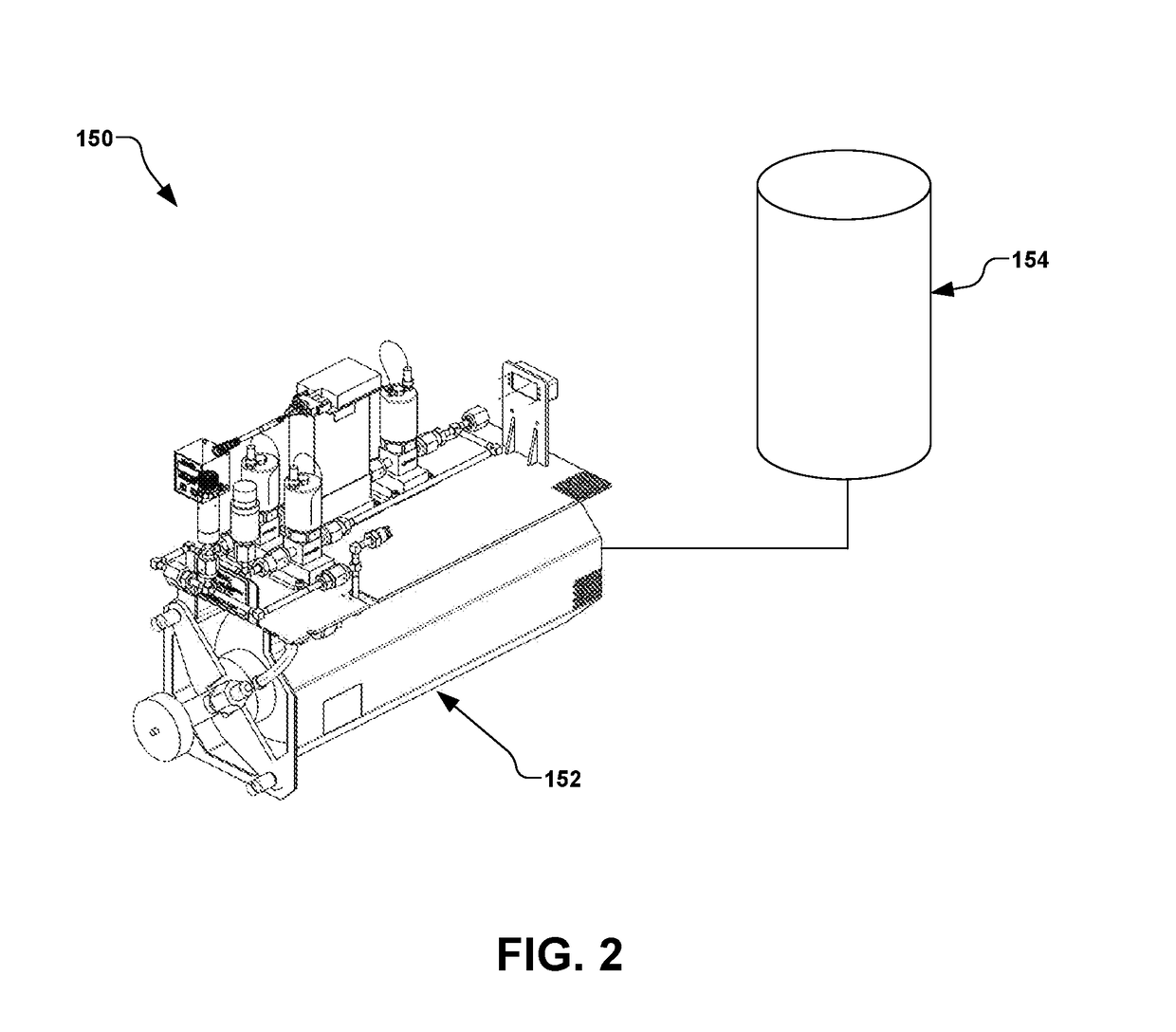
Manufacture of water chemistries
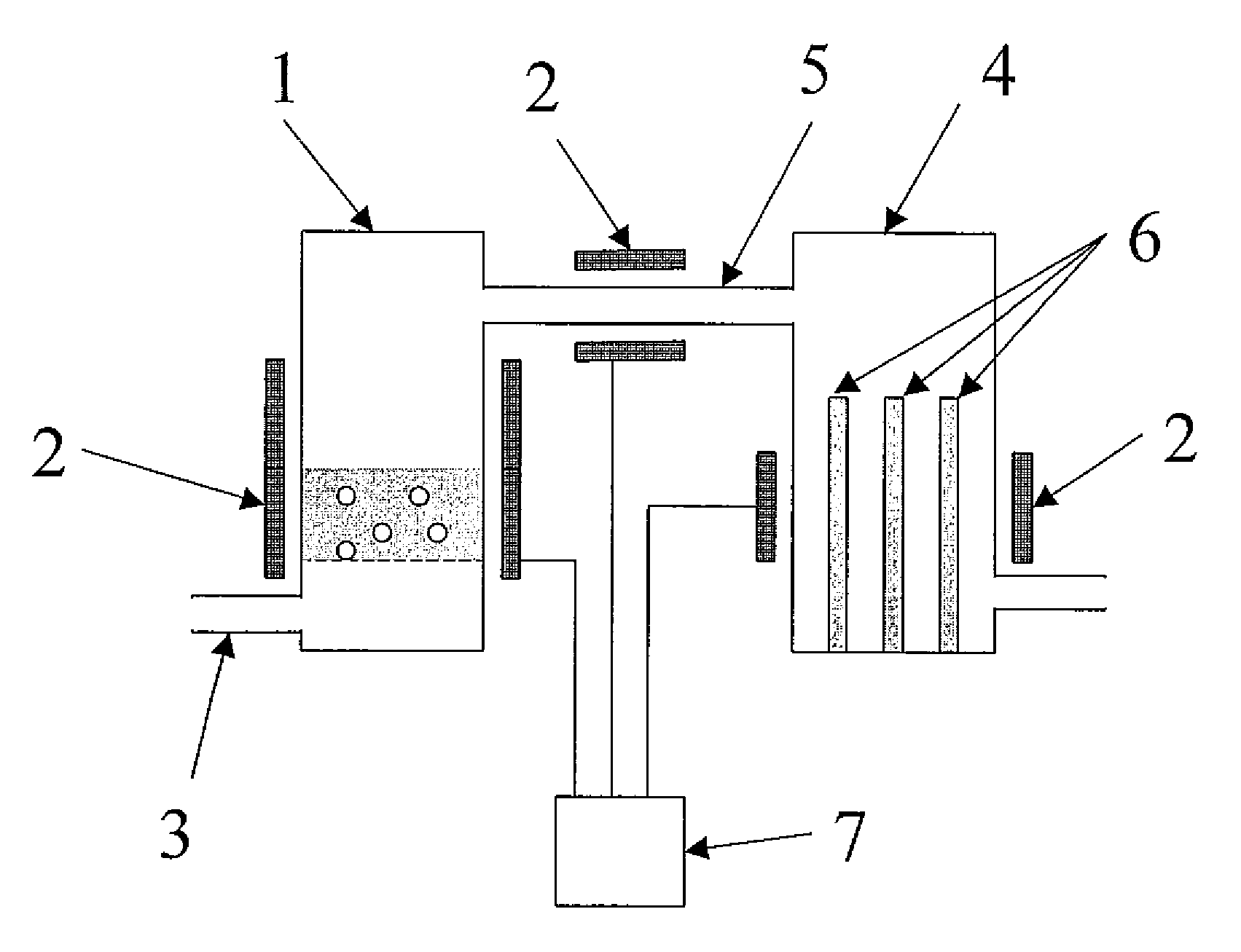
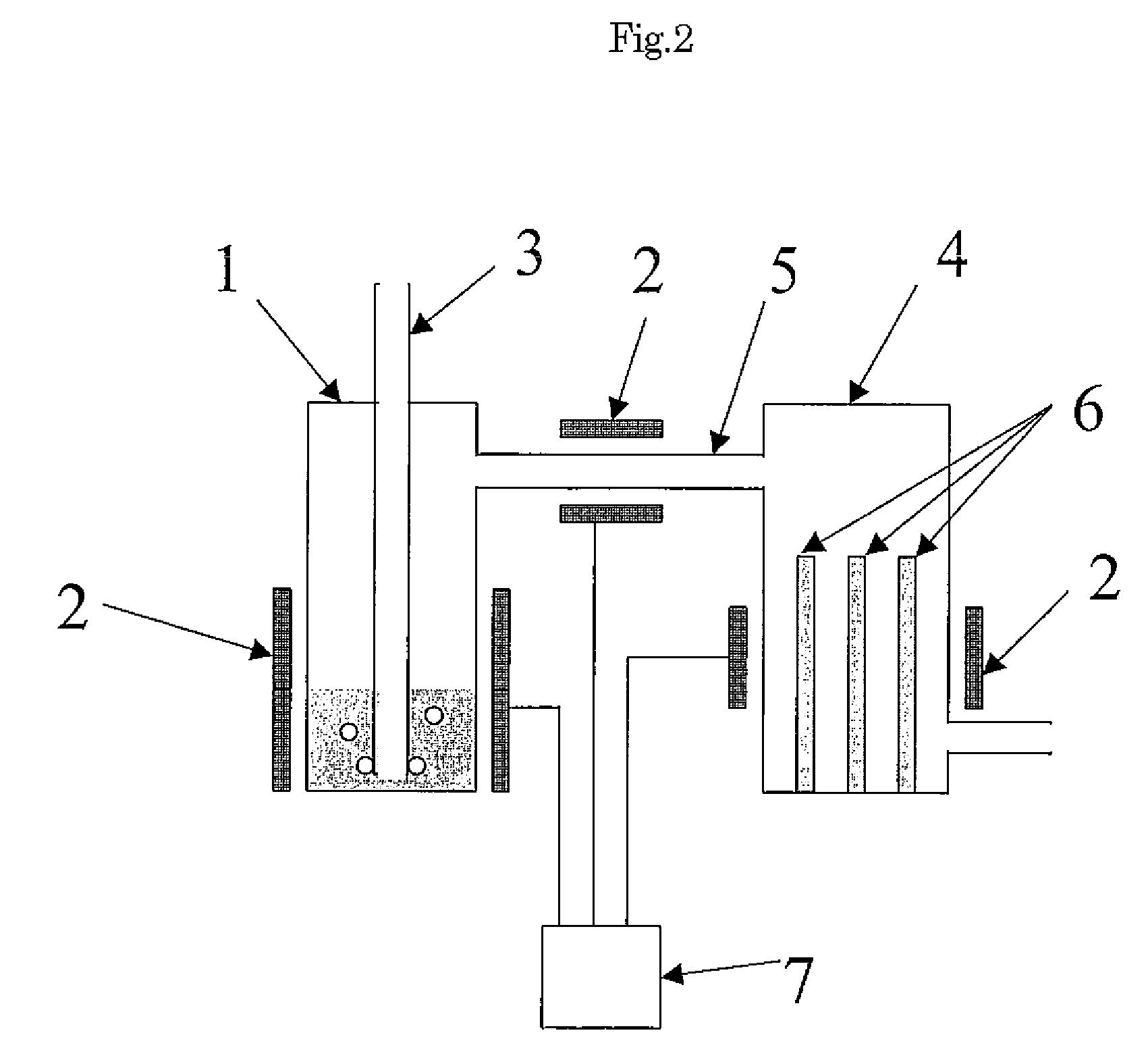
InactiveUS8268269B2Efficient and effective processHigh purityNitrogen compoundsElectrostatic separationPresent methodDisinfectant
As population density increases, the transportation of hazardous chemicals, including acids and disinfectants, lead to an increased incidence of spills while the consequences of spills become more serious. While solutions of halide acids, hypohalites and halites are safer disinfectants for transportation, handling, storage and use than traditional gaseous chlorine, the manufacturing cost of these disinfectants has here-to-fore limited their use. Economical processes are presented for the manufacture of O2, halogen oxides, halide acids, hypohalites, and halates; as well as polynucleate metal compounds, metal hydroxides and calcium sulfate hydrate (gypsum). The instant invention presents methods and processes that incorporate the use of sulfur. This is while environmental regulators, such as the US EPA, require an increased removal of sulfur from hydrocarbon fuels, thereby creating an abundance of sulfur, such that the refining industry is in need of a way to dispose of said abundance of sulfur.
Owner:CLEARVALUE TECH
Method for producing polycrystalline silicon
InactiveUS8173094B2Efficient reductionHigh puritySiliconFinal product manufactureMetal chlorideBoiling point
Owner:SUMITOMO CHEM CO LTD
Process for producing lithium-containing composite oxide for positive electrode for lithium secondary battery
ActiveUS8287828B2Improve featuresSolve the small densityMagnesium halidesCell electrodesAlkaline earth metalNiobium
A process for producing a lithium-containing composite oxide for a positive electrode active material for use in a lithium secondary battery, the oxide having the formula LipQqNxMyOzFa (wherein Q is at least one element selected from the group consisting of titanium, zirconium, niobium and tantalum, N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, alkaline earth metal elements and transition metal elements other than Q and N, 0.9≦̸p≦̸1.1, 0≦̸q<0.03, 0.97≦̸x≦̸1.00, 0≦̸y<0.03, 1.9≦̸z≦̸2.1, q+x+y=1 and 0≦̸a≦̸0.02), which comprises firing a mixture of a lithium, Q element source and N element sources, and an M element source and / or fluorine source when these elements are present, in an oxygen-containing atmosphere, wherein the Q element source is a Q element compound aqueous solution having a pH of from 0.5 to 11.
Owner:SUMITOMO CHEM CO LTD
Method for producing cyclic silane using concentration method
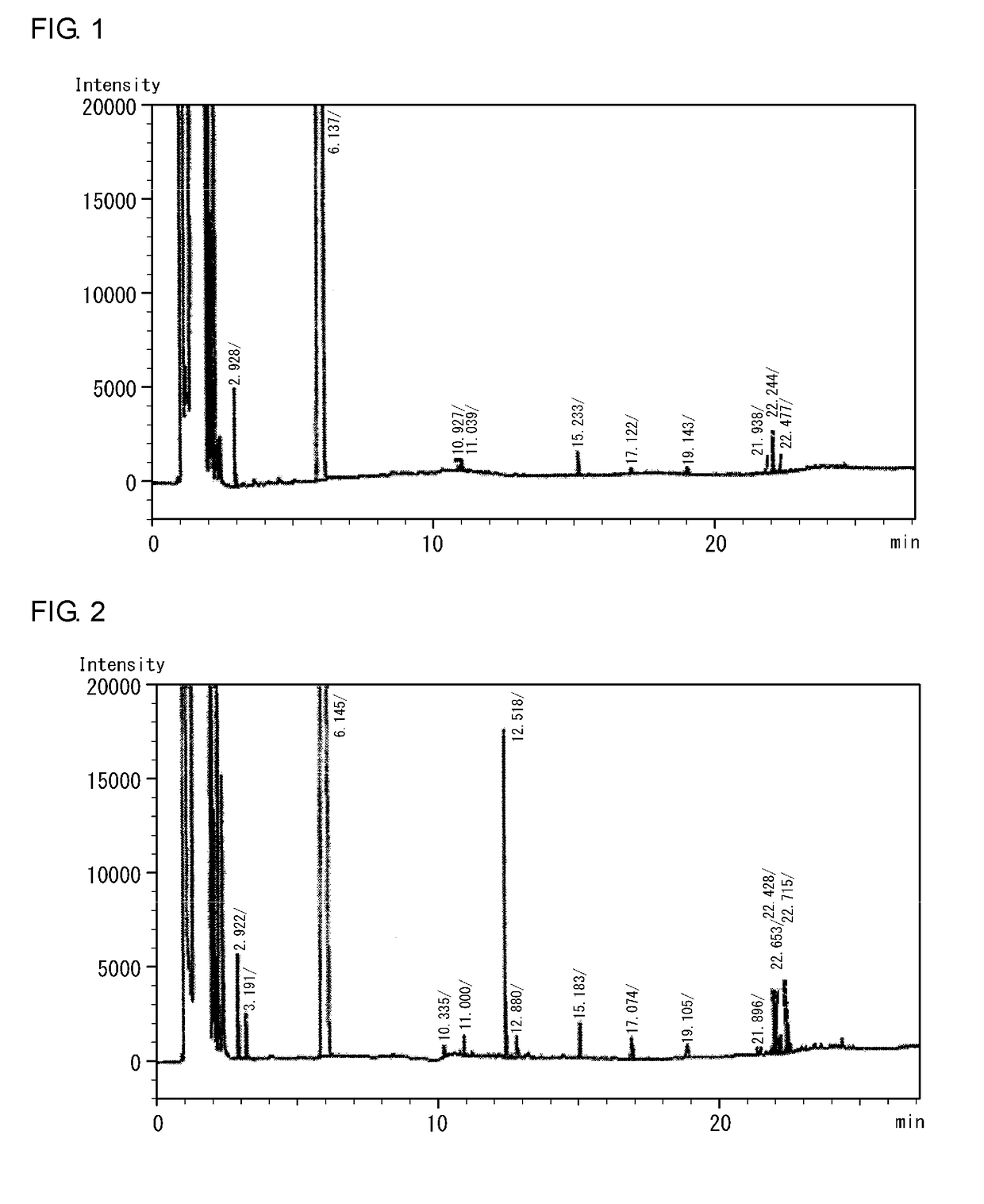
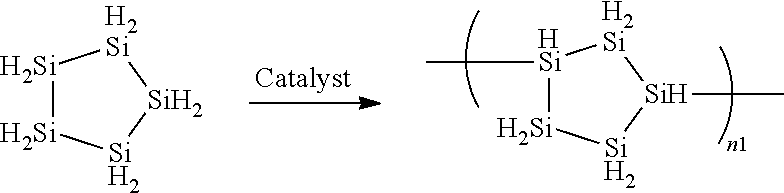
ActiveUS10202283B2High purityEasy to disassembleSilicon hydridesAluminium halidesHydrogen halideDistillation
A cyclic silane having high purity, a composition containing a polysilane obtained by polymerization of the cyclic silane, and a silicon thin film are disclosed. A method for producing a cyclic silane of the formula (SiH2)n, where n is an integer of 4 to 6, includes reacting a cyclic silane compound of the formula (SiR1R2)n (where R1 and R2 are each a hydrogen atom, a C1-6 alkyl group, or a substituted or unsubstituted phenyl group) with a hydrogen halide in the presence of an aluminum halide to obtain a cyclic silane of the formula (SiR3R4)n (where R3 and R4 are each a halogen atom), and then distilling the solution, and reducing the cyclic silane of the formula (SiR3R4)n with hydrogen or lithium aluminum hydride. The distillation may be carried out at a temperature of 40° C. to 80° C. under a reduced pressure of 0 to 30 Torr.
Owner:ENSURGE MICROPOWER ASA
Removal of contaminants from by-product acids
InactiveUS20080267856A1Effective treatmentProvide usageChlorine/hydrogen-chloride purificationSilicaPhosphate ionTitanium
The present invention is drawn to a method for removing colloidal titanium dioxide and titanium oxychloride from by-product hydrochloric acid. The method includes adding phosphate ion source and quaternary amine to the by-product acid to cause the titanium dioxide and the titanium oxychloride to form a precipitate. The precipitate can then be separated from the acid, thus producing a decontaminated hydrochloric acid product with reduced levels of titanium.
Owner:HAYDOCK CONSULTING SERVICES LC
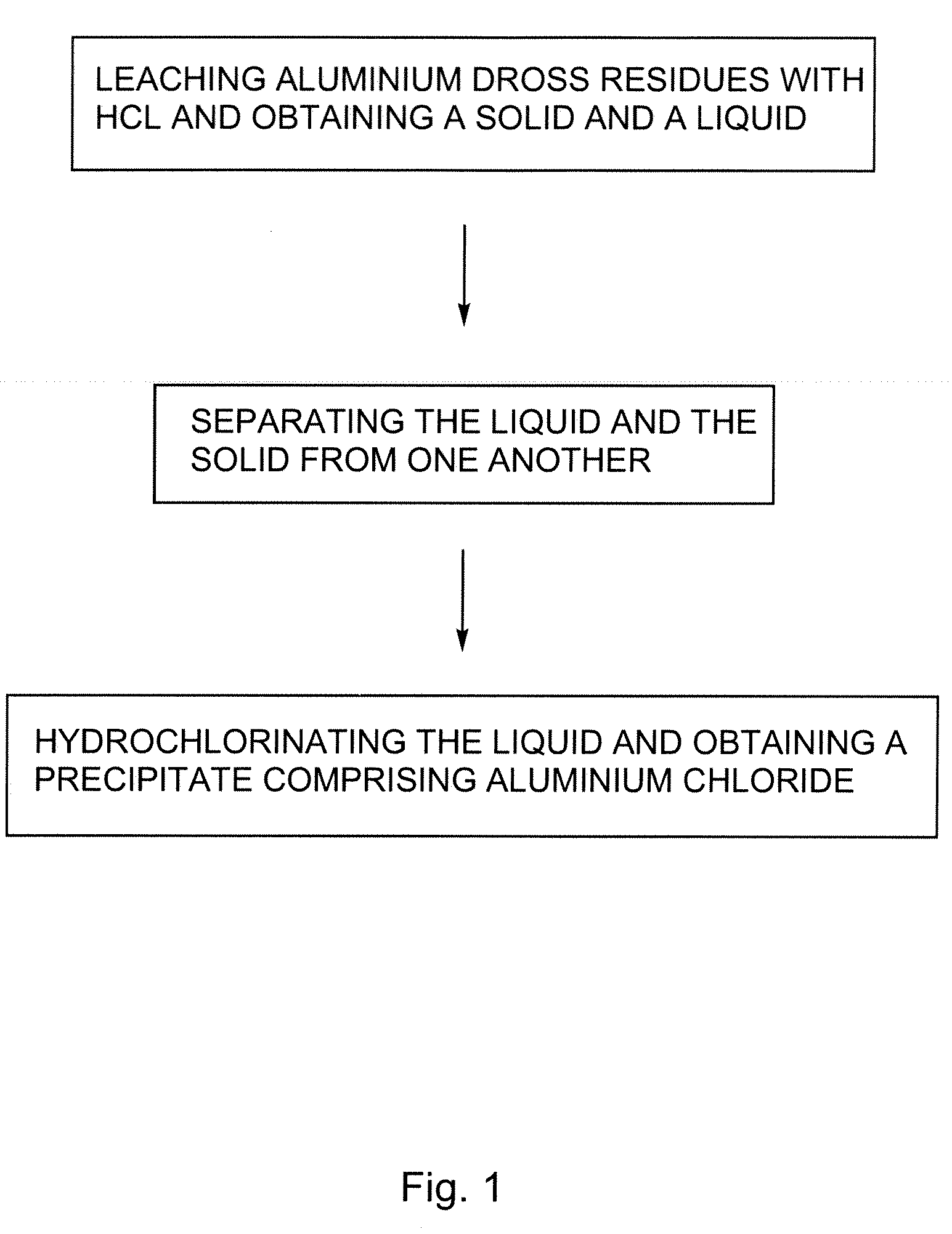
Processes for treating aluminium dross residues
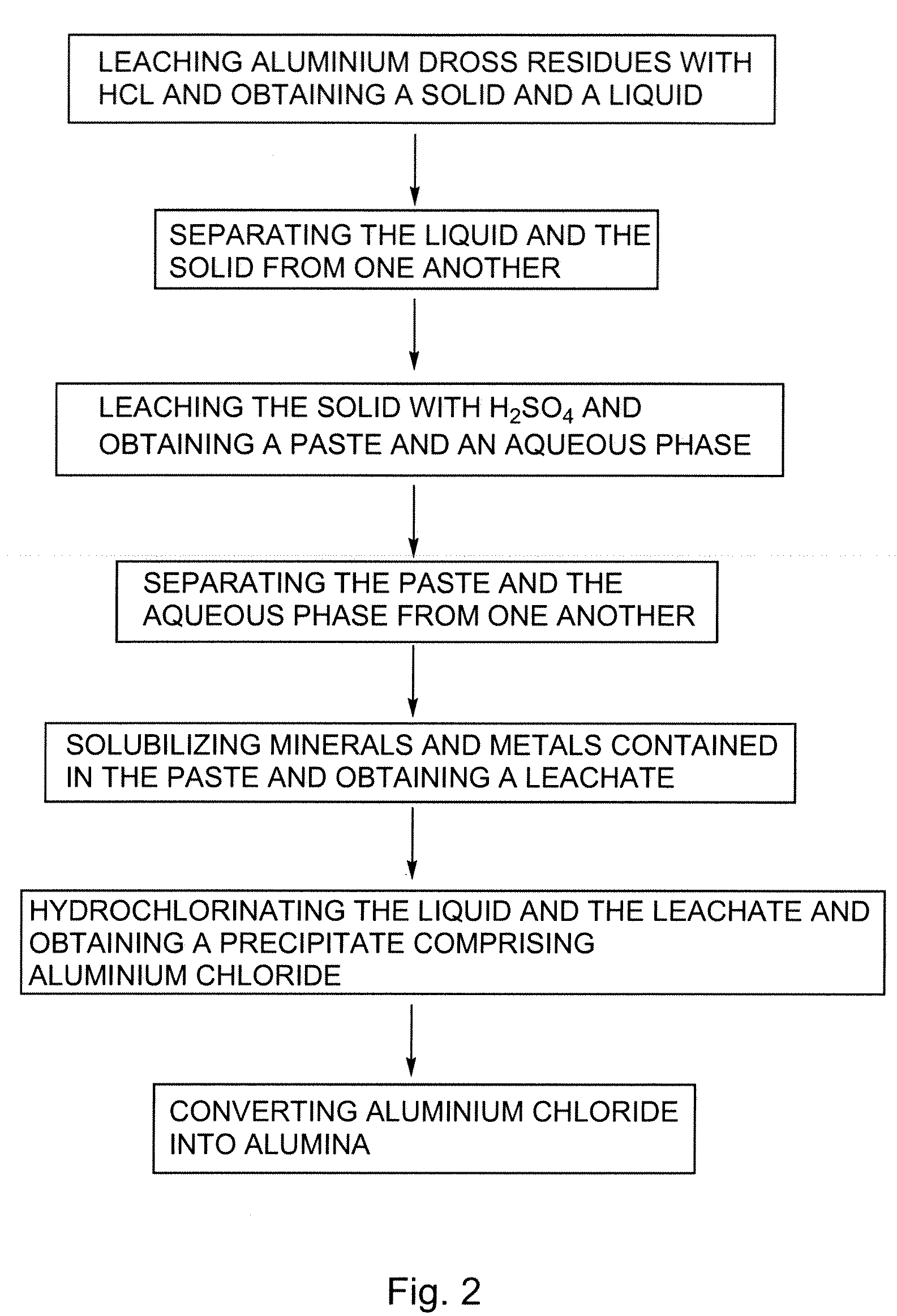
InactiveUS20080159935A1Low costHigh yieldSolvent extractionGallium/indium/thallium compoundsAluminium chlorideLeachate
There is provided a process for preparing aluminium chloride comprising: leaching aluminium dross residues with HCl so as to obtain a mixture comprising a solid and a liquid; and hydrochlorinating the liquid obtained from the mixture, so as to form a precipitate comprising aluminium chloride. Such a sequence can also be used for preparing alumina. In such a case, the process can further comprise the step of converting the so-obtained aluminium chloride into alumina. In the processes previously defined, the solid so-obtained can also be leached with H2SO4, thereby generating a leachate. The leachate can also eventually be hydrochlorinated so as to increase the yield of the desired product obtained i.e. alumina or aluminium chloride.
Owner:EXP SERVICES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com