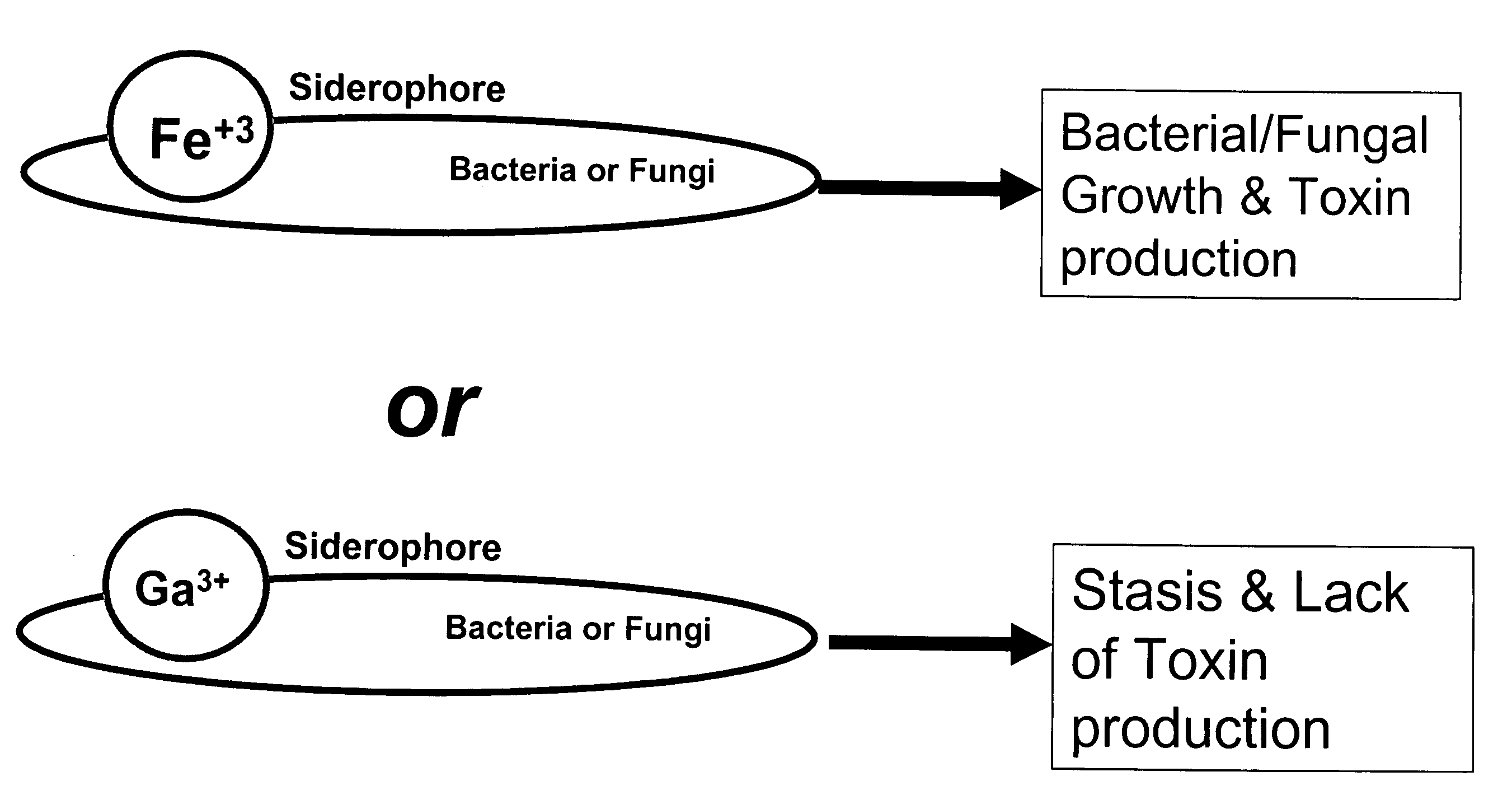
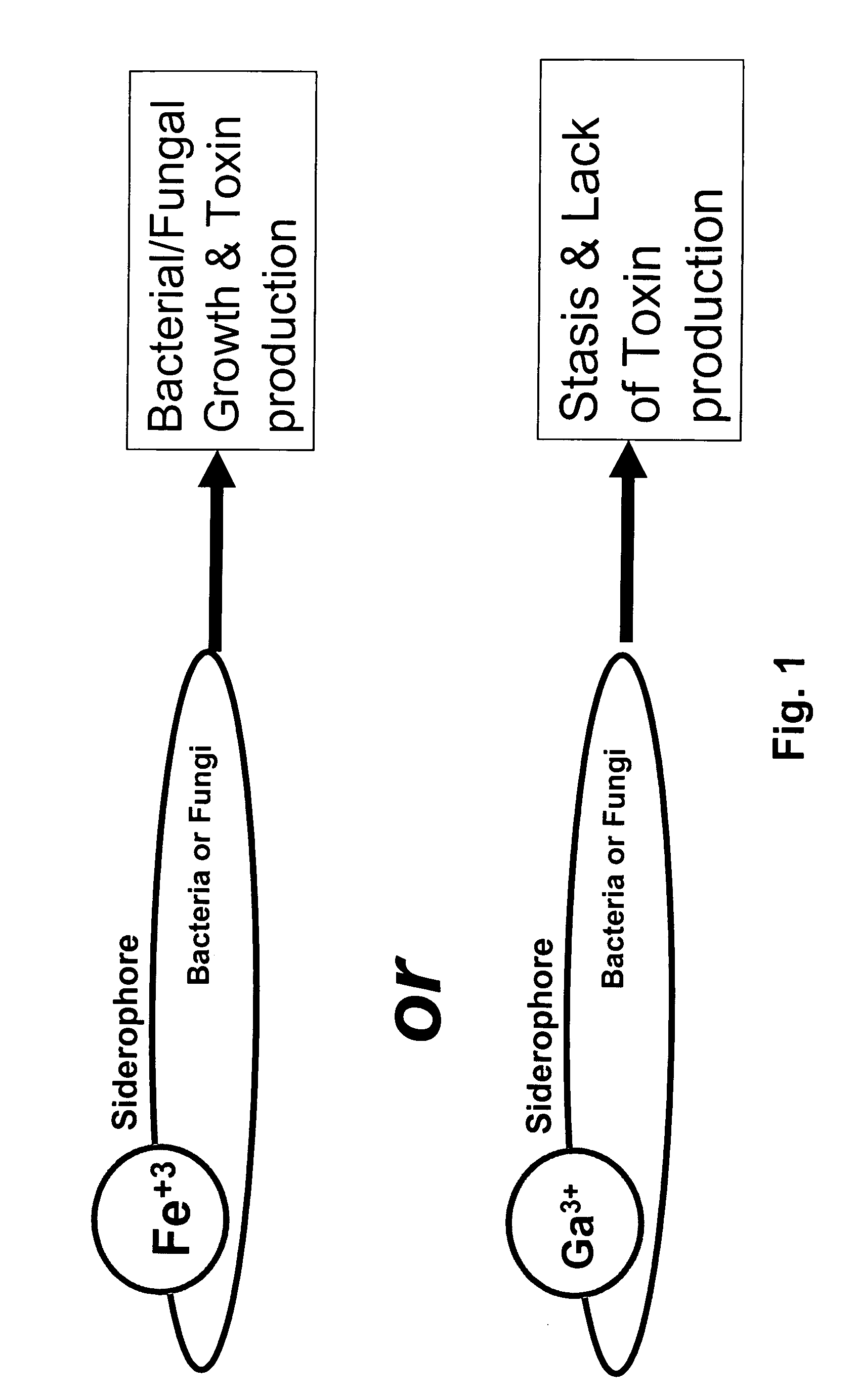
Growth control of oral and superficial microorganisms using gallium compounds
a gallium compound and microorganism technology, applied in the field of growth control of oral and superficial microorganisms using gallium compounds, can solve the problems of gallium entering microorganisms, interfering with the metabolism of organisms, and dna and protein synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Toothpaste Formulation
[0068]A toothpaste is formulated to contain gallium nitrate at 50 μM and 0.2% by weight sodium fluoride as active ingredients in the treatment and prevention of dental and periodontal disease. The formulation also contains conventional inactive ingredients including water, sorbitol, glycerin, hydrated silica, polyethyleneglycol, xanthan gum, sodium saccharin, methylparaben, and propylparaben. These are blended in amounts known to those skilled in the art to obtain a toothpaste of appropriate consistency. The formulation can be packaged in conventional toothpaste tubes, and used in the normal manner.
example 2
Treatment of Vaginal Yeast Infection
[0069]The present invention is applied to the formulation of suppositories and creams for the treatment of internal and external vaginal yeast infections, respectively, as follows. Gallium citrate at 200 μM is combined with inactive ingredients including, in the case of a suppositories, gelatin, glycerin, lecithin, mineral oil, titanium oxide, and white petrolatum, or, in the case of external creams, benzoic acid, isopropylmyristate, polysorbate 60, potassium hydroxide, propyleneglycol, distilled water and stearyl alcohol. These formulations can be suitably packaged for use in a conventional manner.
example 3
Disinfection of Inanimate Objects
[0070]A liquid formulation in a form of an aqueous solution of 5% by weight gallium phosphate and 5% by weight dimethyl benzyl ammonium chloride is prepared and packaged for use in households, hospitals, barber shops and beauty parlors as a surface treatment agent for disinfecting objects whose surfaces are prone to acting as sites for the growth of pathogenic organisms. Such a formulation can be used by application to such surfaces in a conventional manner.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com