Method for preparing polypyrrole monovalent selective cation exchange membrane in situ
A cation exchange membrane and a technology for preparing polypyrrole, applied in the field of ion exchange membrane, can solve the problems of short service life, unfriendly environment, complicated preparation process, etc., and achieve the effects of improving stability, effective separation, and simple and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
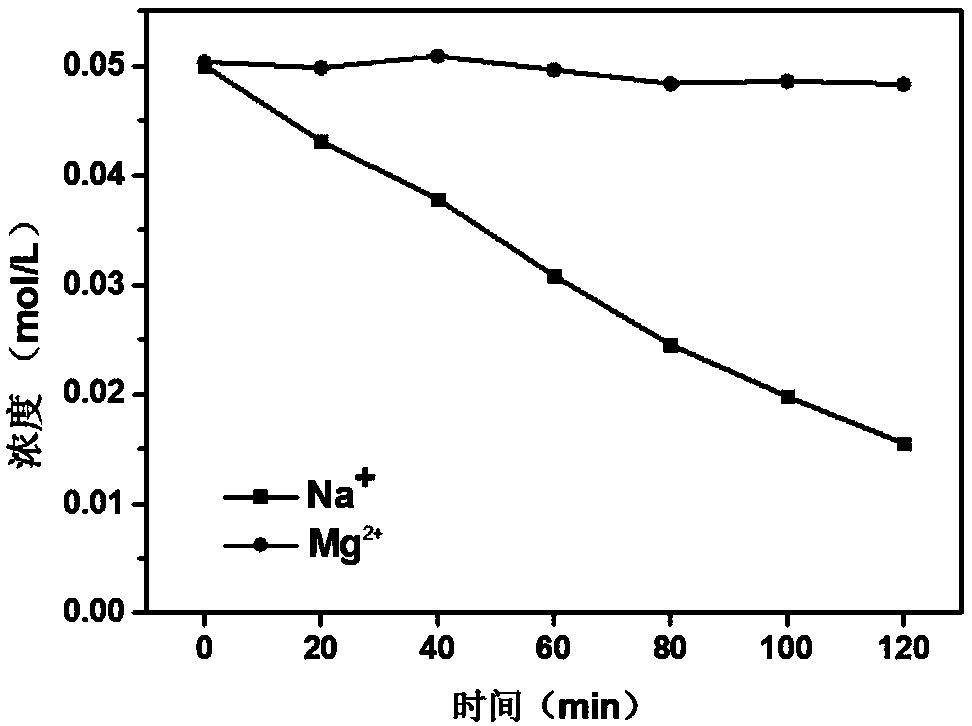
[0027] (1) the common cation exchange membrane (white, 50cm 2 ) after soaking in 0.2mol / L hydrochloric acid solution for 2 hours, wash off the acid on the surface with deionized water. (2) Soak the hydrogen-type cation exchange membrane in 4wt% pyrrole alcohol solution (200ml) for 1 minute, take out the membrane, and place it horizontally for more than 2 minutes. After the surface ethanol evaporates completely, immerse the membrane in 0.5mol / L trichloro After 3 hours in the ferric chloride solution, the film turned black at this time, which proved that polypyrrole had been loaded on the film. (3) Repeat step (2) 3 times to obtain a selective cation exchange membrane polymerized 4 times. Wash the membrane surface repeatedly with pure water and ethanol solution to ensure that there is no residual ferric chloride solution and monomeric pyrrole.
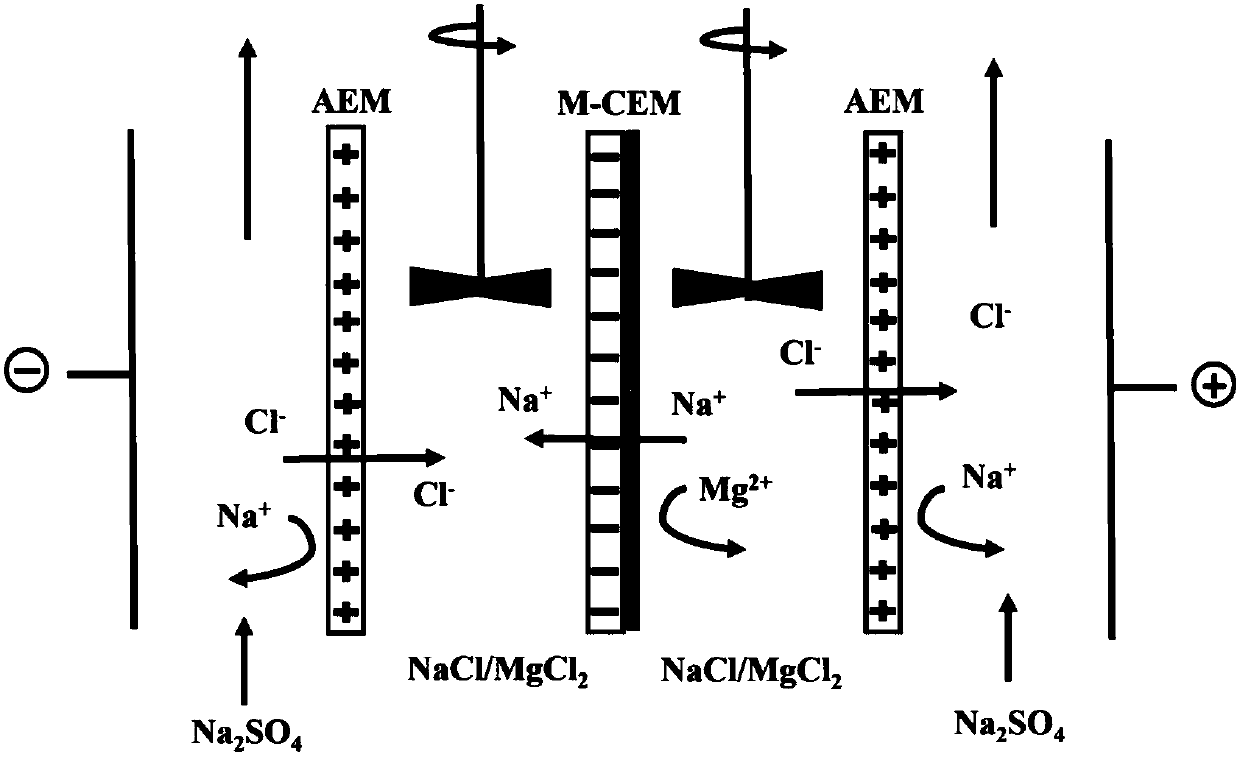
[0028] The membrane was placed in a homemade four-compartment electrodialysis unit, such as figure 1 As shown, M-CEM represents the ...
Embodiment 2
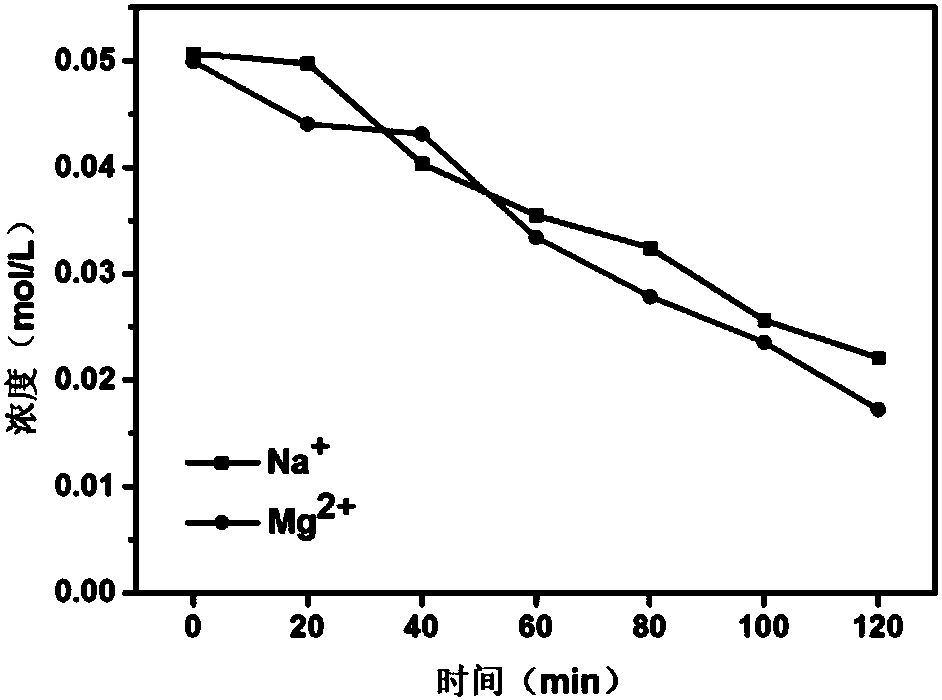
[0030] The effect of polypyrrole concentration on the separation performance was investigated. (1) the common cation exchange membrane (white, 50cm 2 ) was soaked in 0.2mol / L hydrochloric acid solution for 2 hours, and the surface hydrogen ions were washed away with deionized water.
[0031] (2) Soak the acidified cation exchange membrane in 2, 3, 4, 5wt% pyrrole alcohol solution (200ml) for 1 minute respectively, take out the membrane, place it horizontally for more than 2 minutes, after the surface ethanol evaporates completely, remove the membrane Immersed in 0.5mol / L ferric chloride solution for 3 hours, at this time the film was black, which proved that polypyrrole had been loaded on the film. (3) Repeat step (2) 3 times to obtain a selective cation exchange membrane polymerized 4 times. Wash the membrane surface repeatedly with pure water and ethanol solution to ensure that there is no residual ferric chloride solution and monomeric pyrrole.
[0032] The membranes wer...
Embodiment 3
[0034] The effect of polypyrrole modified layers on the separation performance was investigated. (1) the common cation exchange membrane (white, 50cm 2 ) was soaked in 0.2mol / L hydrochloric acid solution for 2 hours, and the surface hydrogen ions were washed away with deionized water. (2) Soak the acidified cation exchange membrane in 4wt% pyrrole alcohol solution (200ml) for 1 minute, take out the membrane, and place it horizontally for more than 2 minutes. After the surface ethanol evaporates completely, immerse the membrane in 0.5mol / L trichloro After 3 hours in the ferric chloride solution, the film turned black at this time, which proved that polypyrrole had been loaded on the film. (3) Step (2) is repeated 1-5 times respectively to obtain selective cation exchange membranes with different times of polymerization. Wash the membrane surface repeatedly with pure water and ethanol solution to ensure that there is no residual ferric chloride solution and monomeric pyrrole. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com