Method and application for preparing fully degradable chitosan hemostatic powder by spray drying process
A technology of spray drying and chitosan, which is applied in the field of medical hemostatic auxiliary materials, can solve cumbersome and time-consuming problems, and achieve the effects of simple operation, convenient preparation, and shortened healing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method of absorbable single-component chitosan blood powder is as follows:
[0029] (1) Weigh 7 grams of chitosan, dissolve it in 1 L, 10 g / L of acetic acid solution, and configure it into a 7 g / L chitosan solution. The pH of the solution was 4.5.
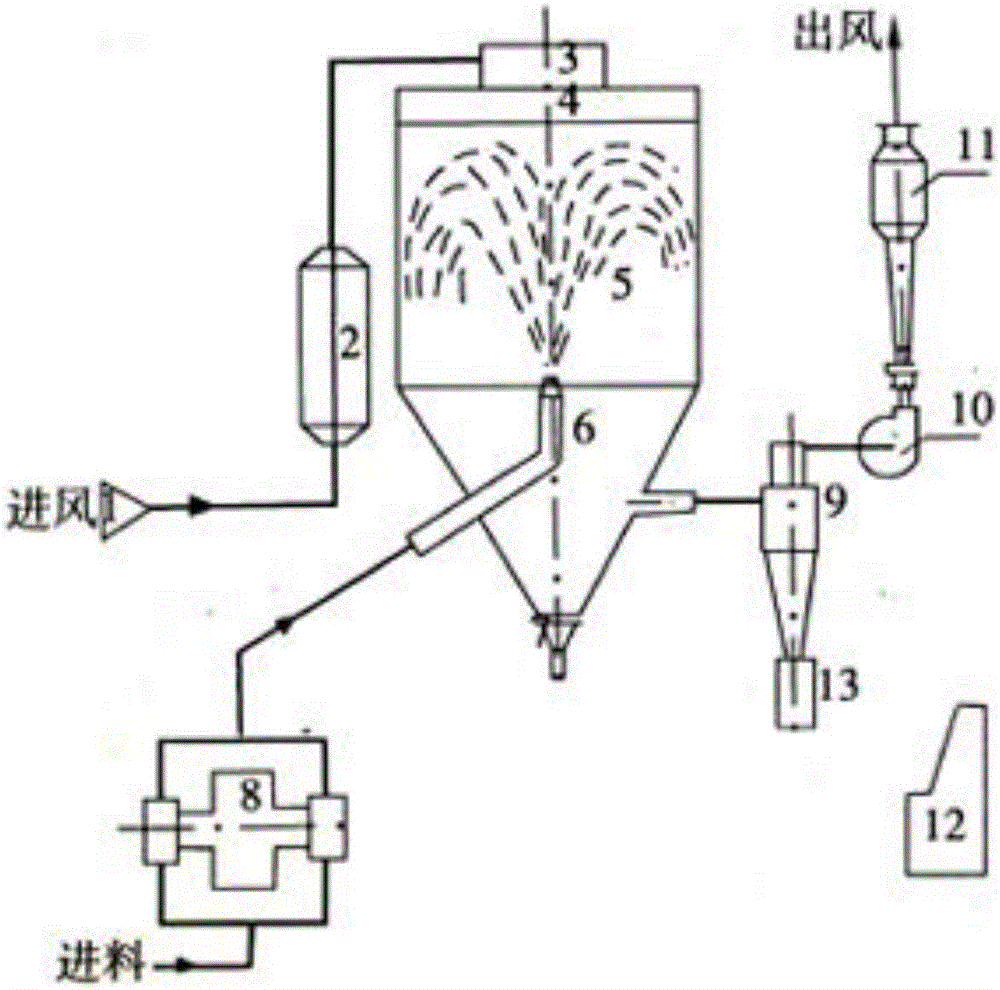
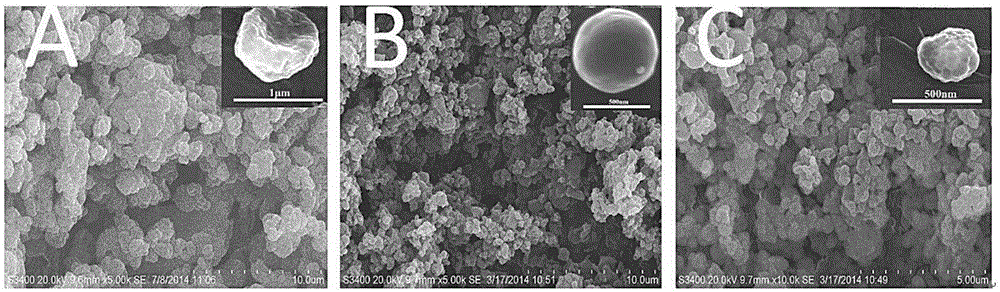
[0030] (2) The chitosan solution of the above-mentioned configuration is spray-dried, adopting the B-290 spray drying instrument of Swiss buchi company, the nozzle diameter is 0.5mm, the inlet temperature is 180°C, the outlet temperature is 105°C, and the spray speed is 5mL / min , The compressed air speed is 10L / min. Collect powder product, yield 80%
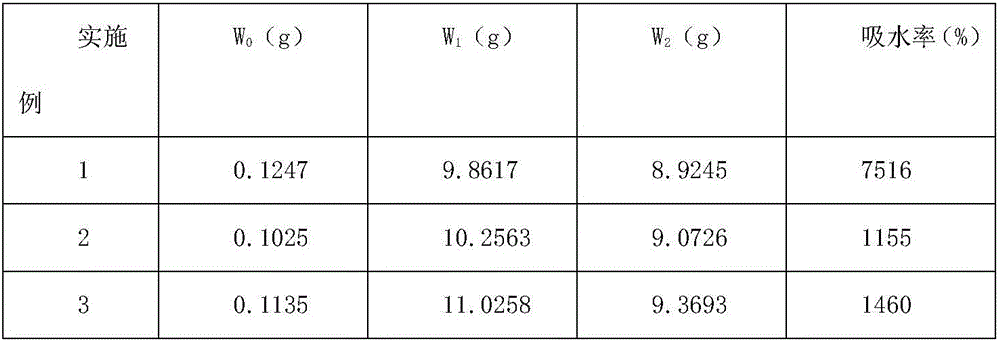
[0031] (3) Configure 1% citric acid solution, and spray evenly on the surface of the above-mentioned powder product after being atomized by a medical nebulizer. Take 0.1 g of the modified powder sample, disperse it in 5 mL of distilled water, and measure the pH value. When the pH value is between 4-5.5, the modification is complete. Store the hemostatic porti...
Embodiment 2
[0033] A preparation method of absorbable single-component chitosan blood powder is as follows:
[0034] (1) Weigh 6 grams of carboxymethyl chitosan, dissolve it in 1 L, 1 g / L of acetic acid solution, and configure it into a 6 g / L chitosan solution. The pH of the solution was 5.0.
[0035] (2) Spray-dry the carboxymethyl chitosan solution configured above, using a B-290 spray dryer from Buchi, Switzerland, with a nozzle diameter of 1mm, an inlet temperature of 160°C, an outlet temperature of 105°C, and a spray speed of 7mL / min, the compressed air speed is 12L / min. Collect powder product, yield 82%
[0036] (3) Configure 1% citric acid solution, and spray evenly on the surface of the above-mentioned powder product after being atomized by a medical nebulizer. Take 0.1 g of the modified powder sample, disperse it in 5 mL of distilled water, and measure the pH value. When the pH value is between 4-5.5, the modification is complete. Store the hemostatic portion after disinfec...
Embodiment 3
[0038] A kind of preparation method of absorbable multicomponent chitosan blood powder is as follows:
[0039] (1) Weigh 7 grams of chitosan, dissolve it in 1 L, 10 g / L of acetic acid solution, and configure it into a 7 g / L chitosan solution. The pH of the solution was 4.5.
[0040] (2) Prepare an aqueous solution of polyvinyl alcohol, whose mass fraction is 1 g / L. The above-mentioned chitosan solution and polyvinyl alcohol aqueous solution are configured into a mixed solution, and the volume ratio: chitosan solution: polyvinyl alcohol solution is 1:1 (volume ratio).
[0041] (3) Spray-dry the mixed chitosan solution of the above-mentioned configuration, adopt the B-290 spray dryer of Buchi Company in Switzerland, the nozzle diameter is 1mm, the inlet temperature is 160°C, the outlet temperature is 105°C, and the spray speed is 10mL / min , The compressed air speed is 12L / min. Collect powder product, yield 90%
[0042] (4) Configure 1% lactic acid solution, and spray evenly ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com