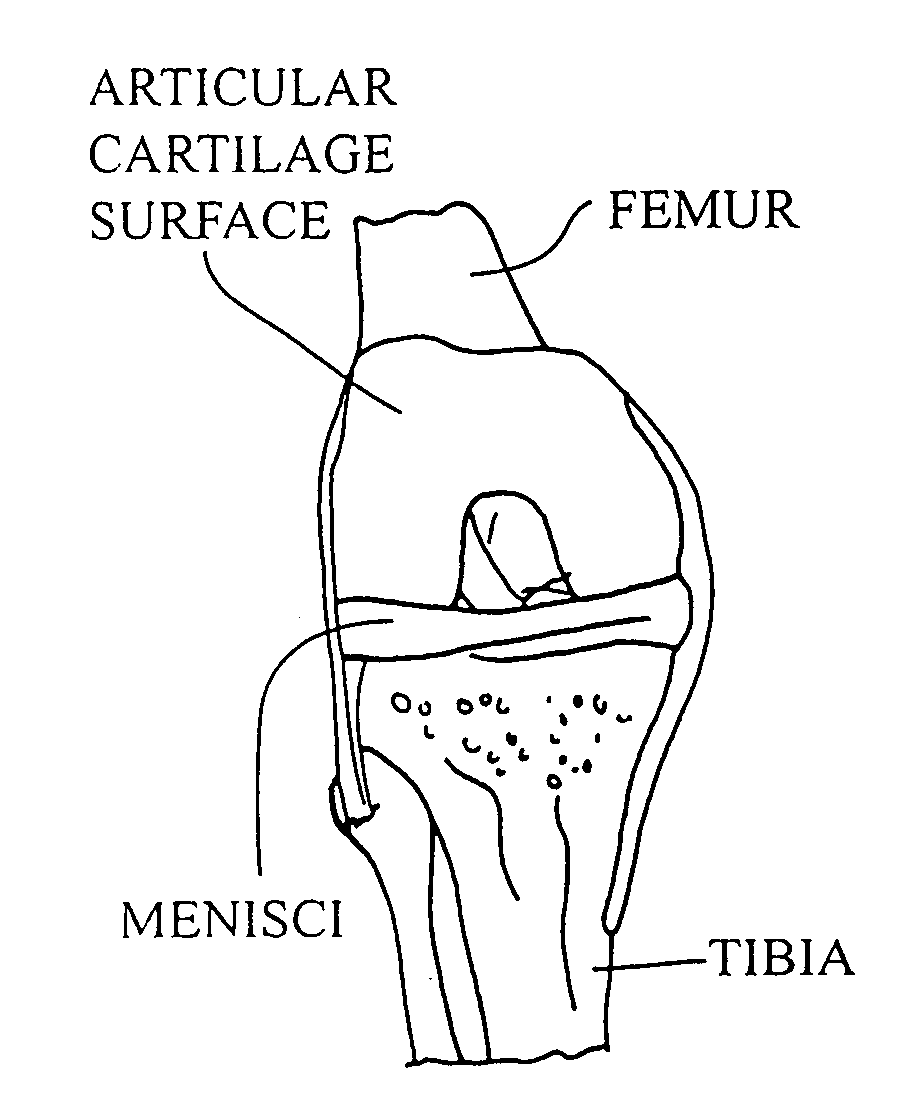
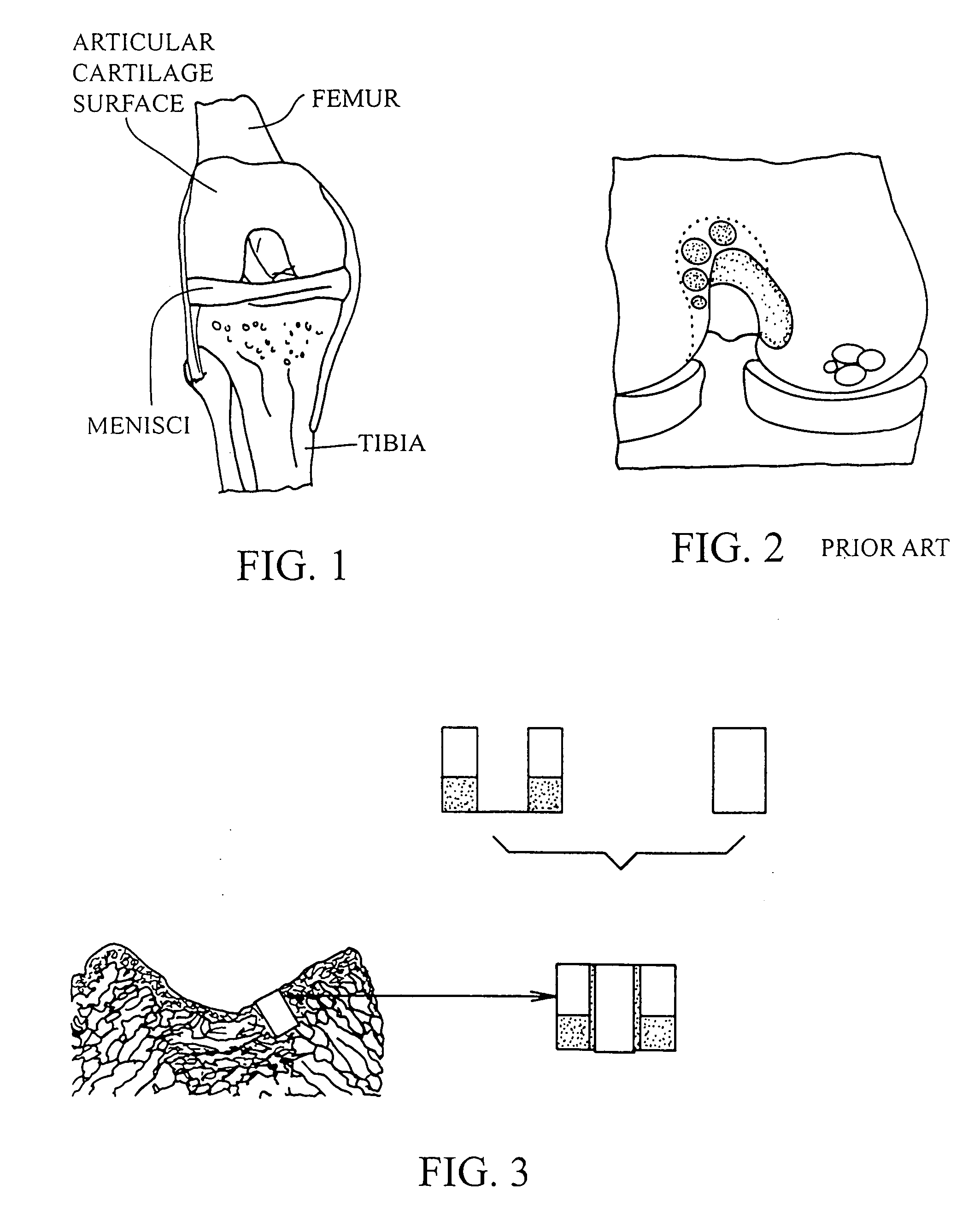
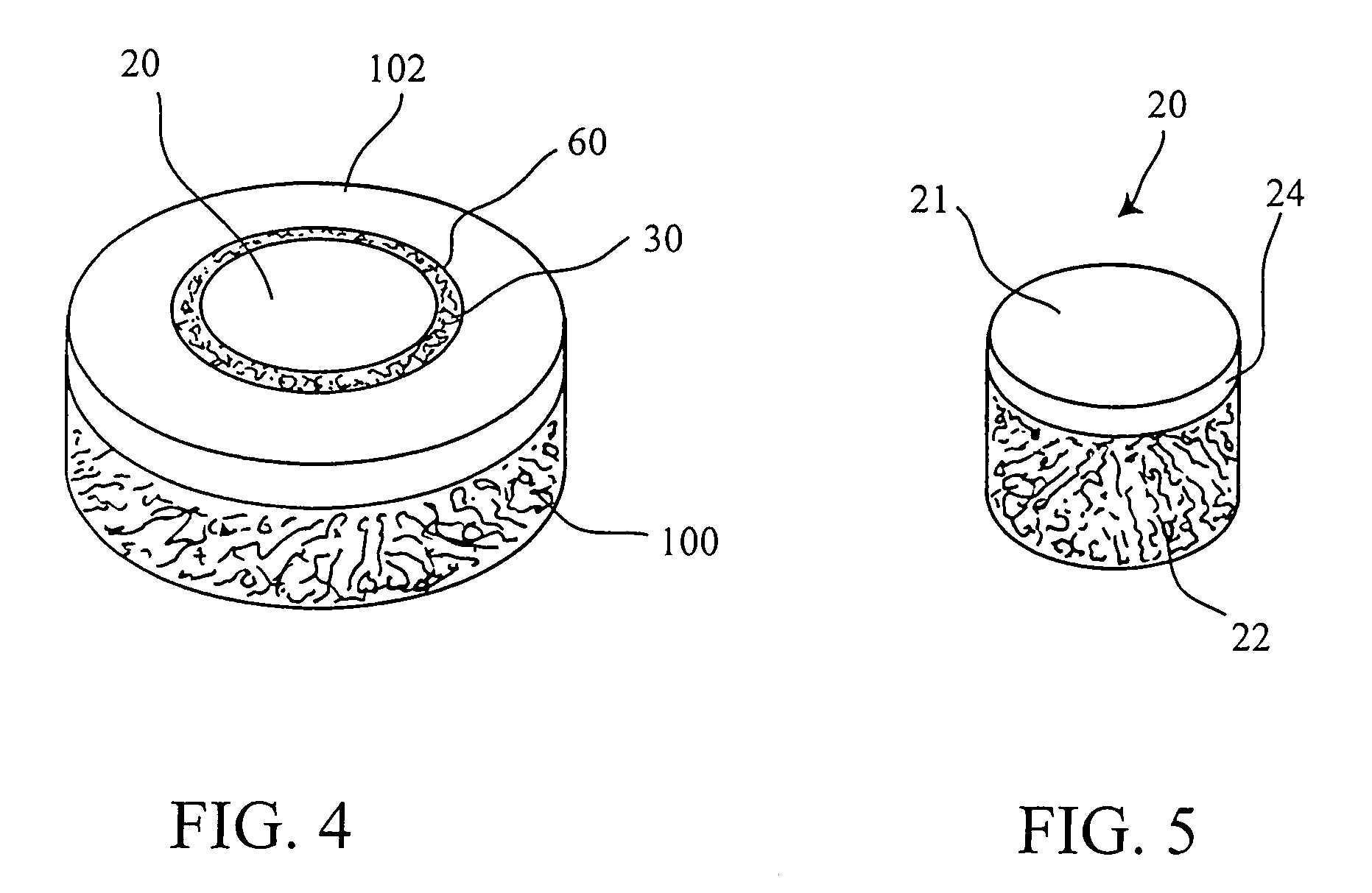
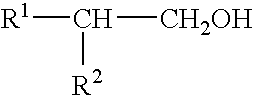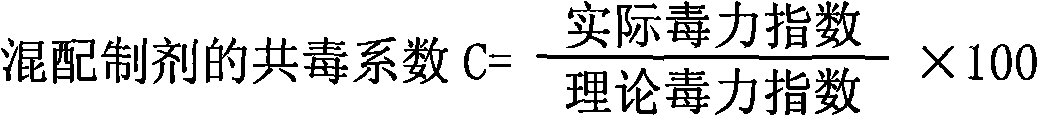Patents
Literature
112 results about "Proteoglycan Core Protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
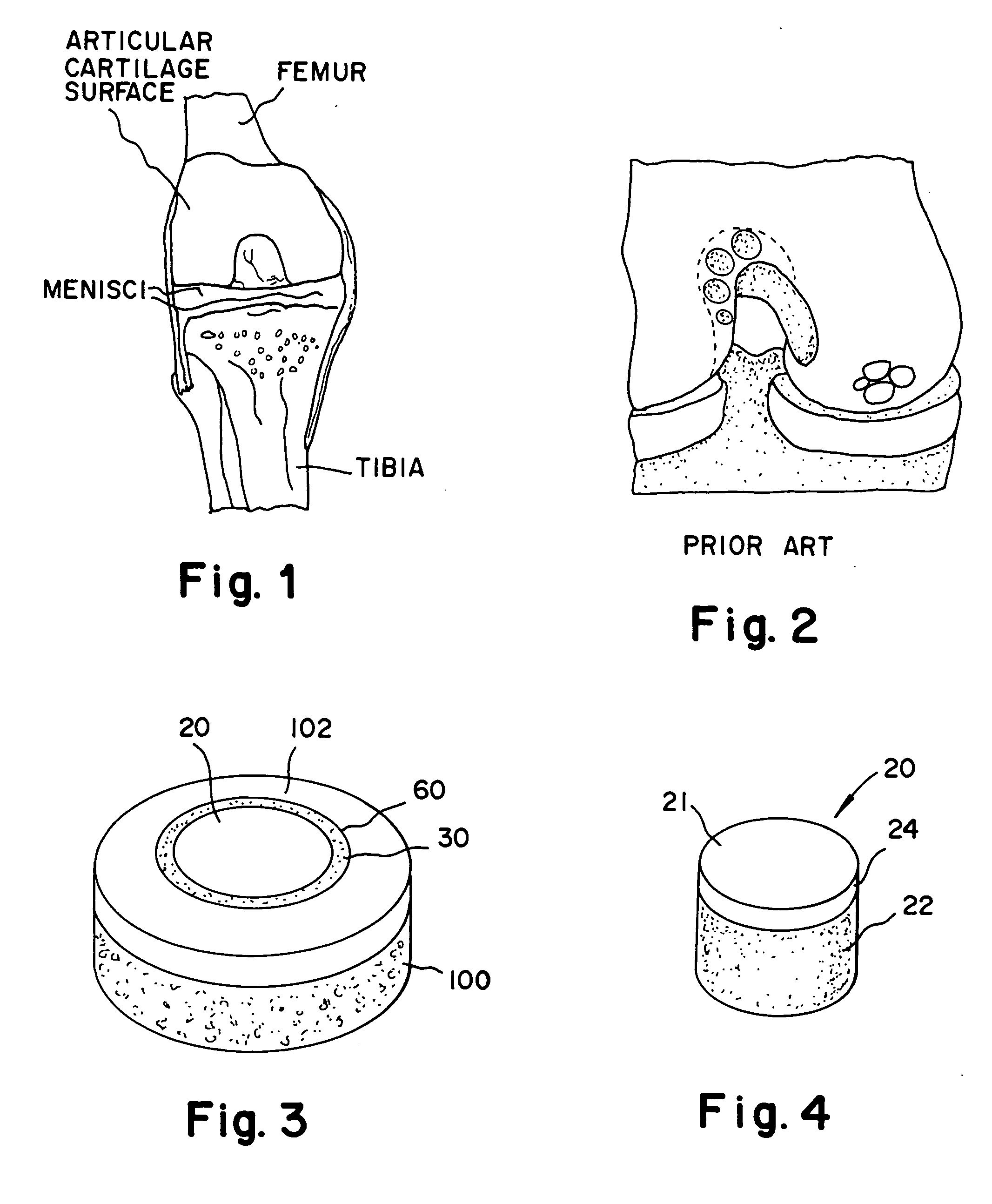
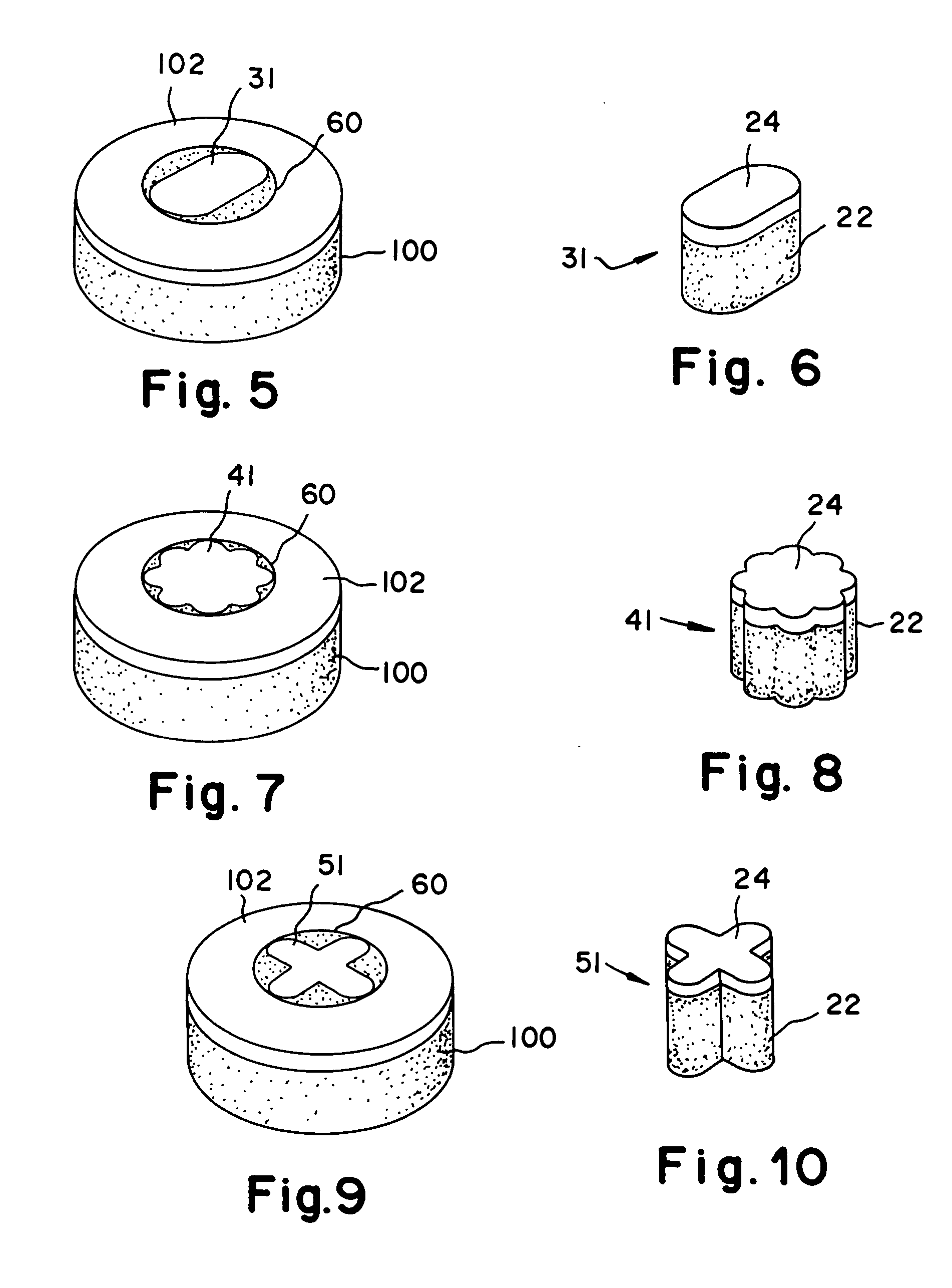
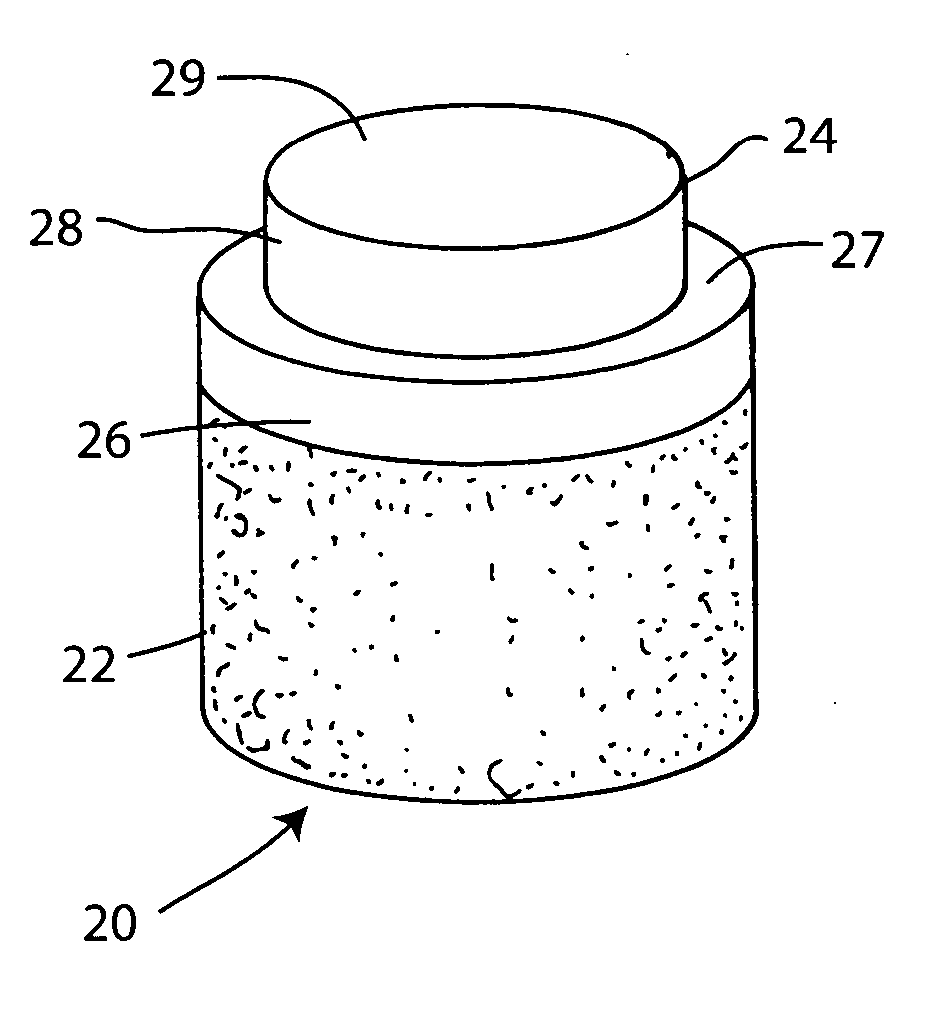
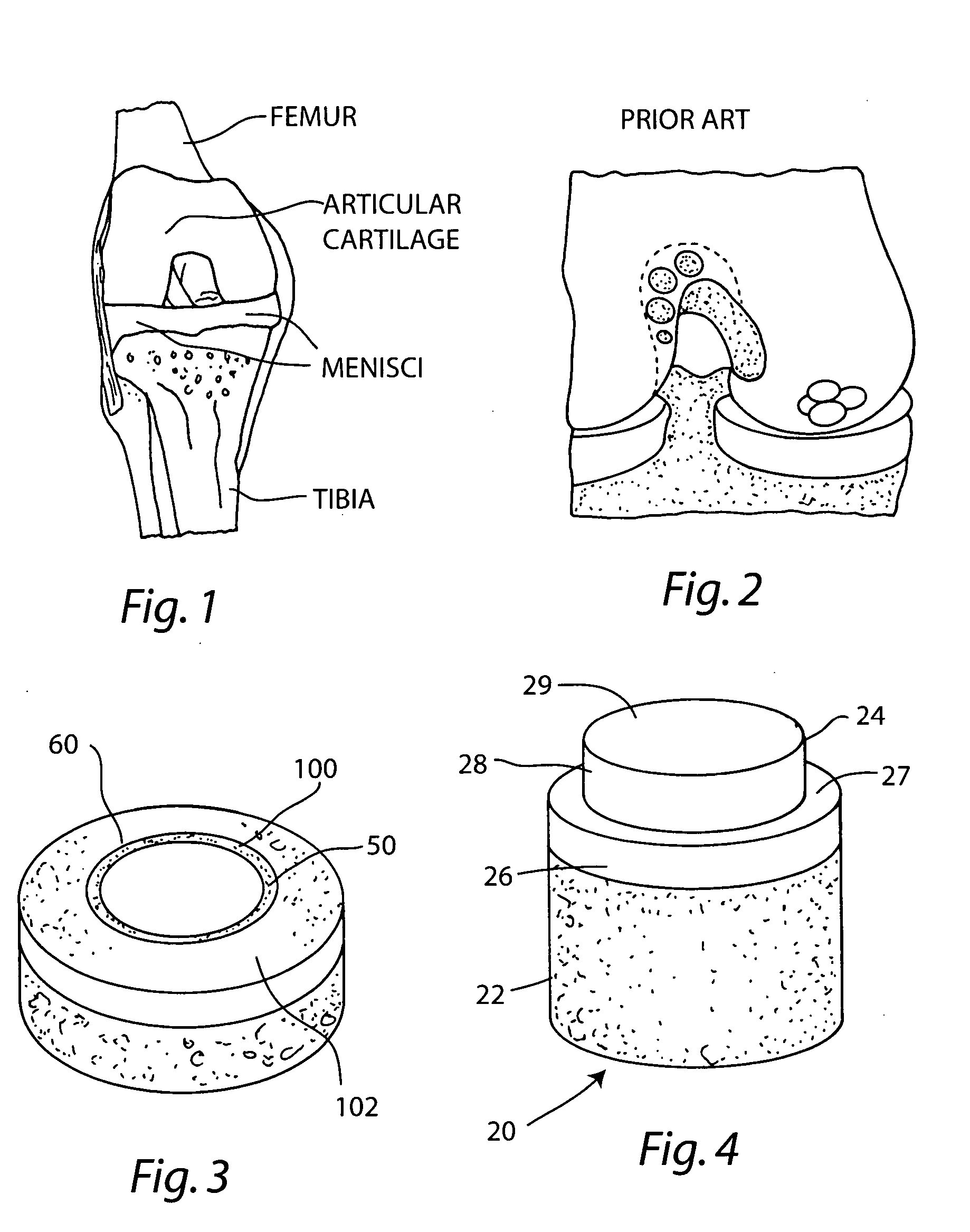
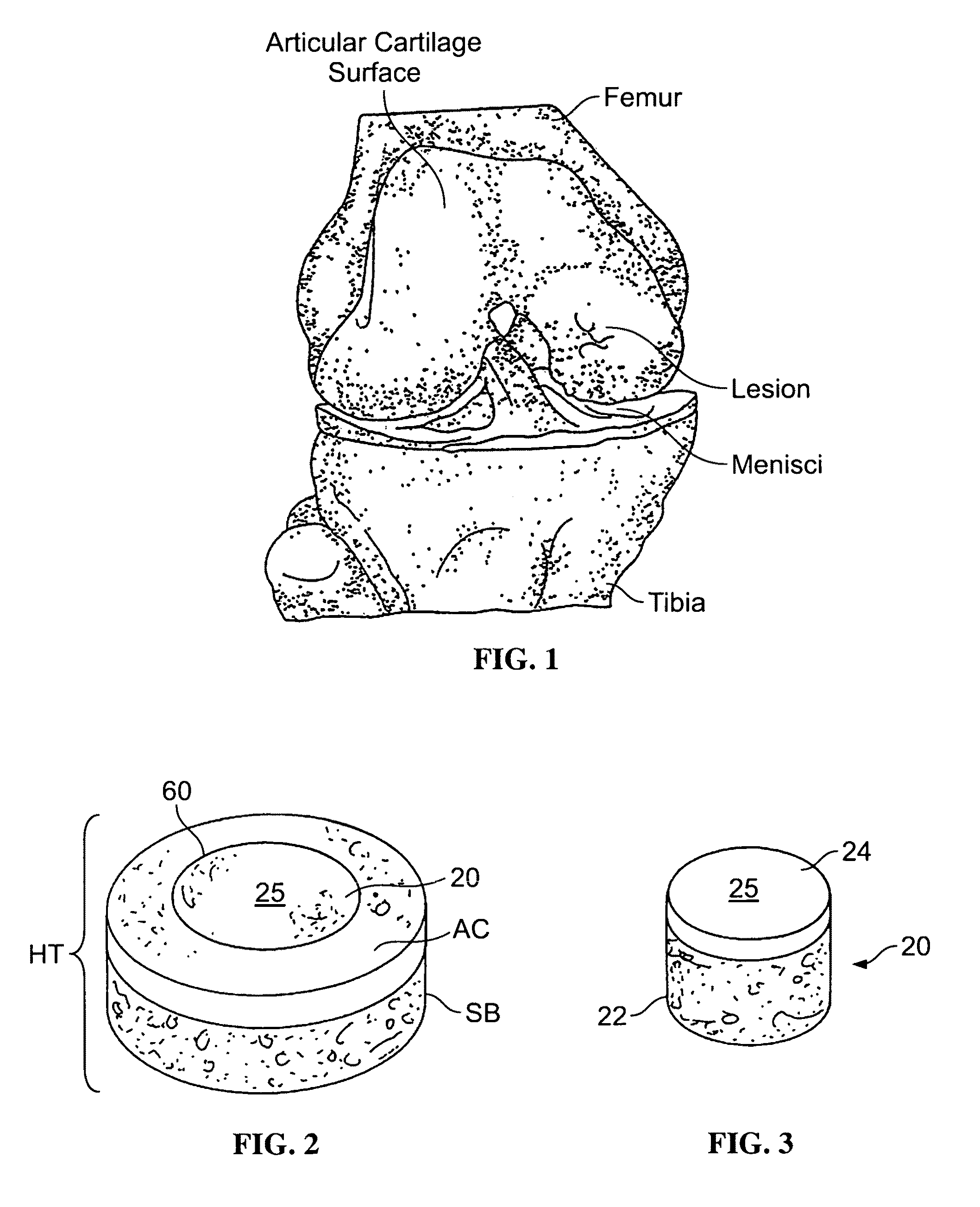
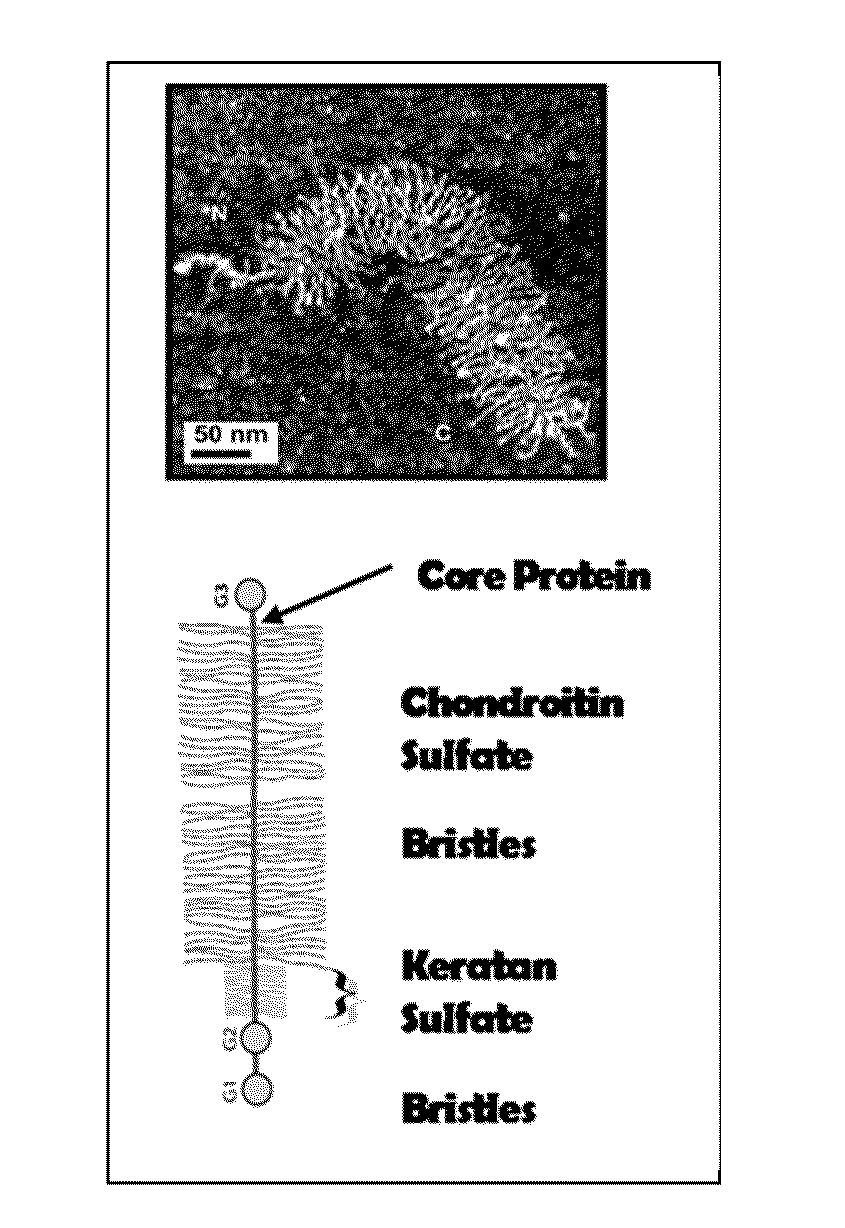
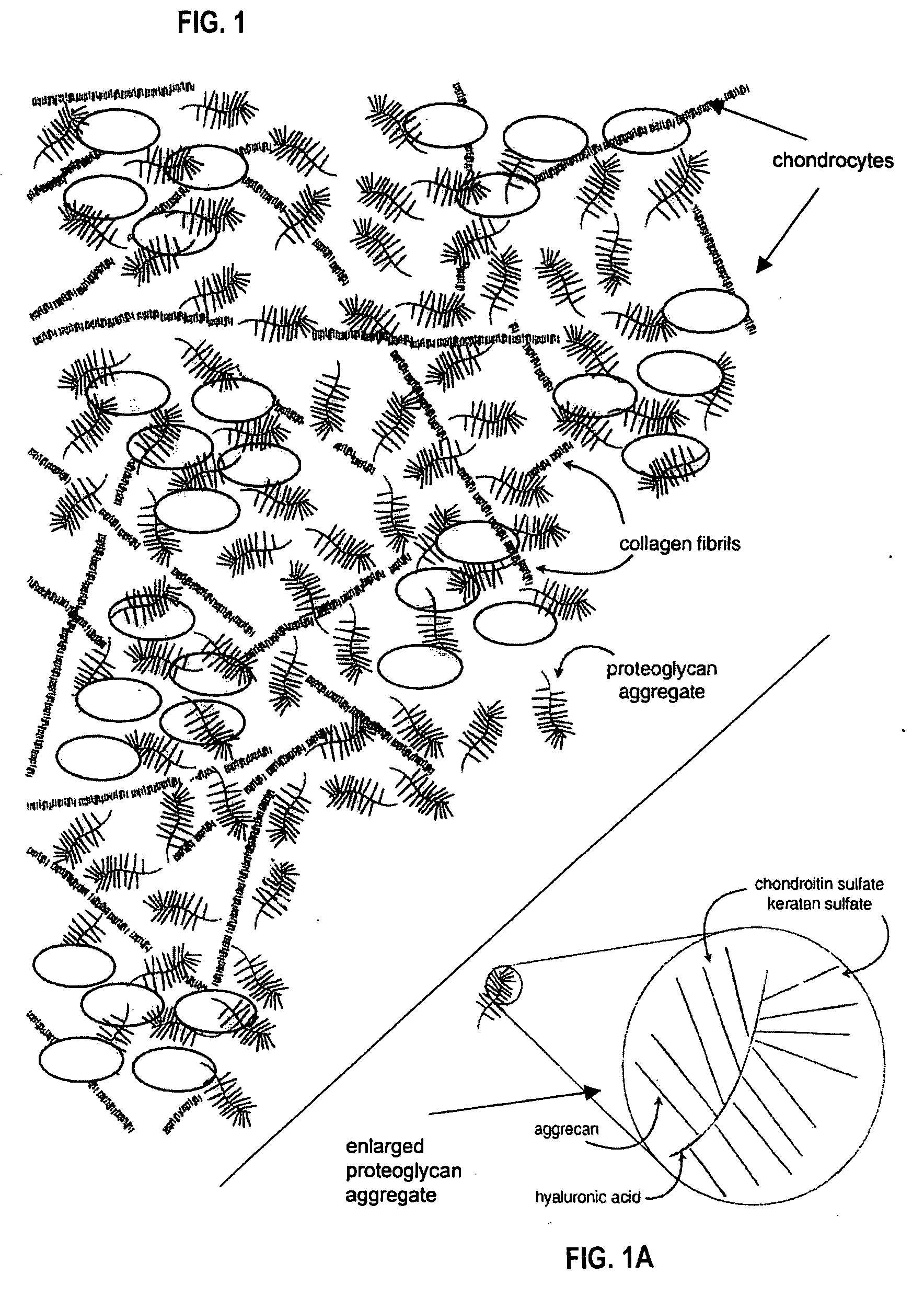
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s).
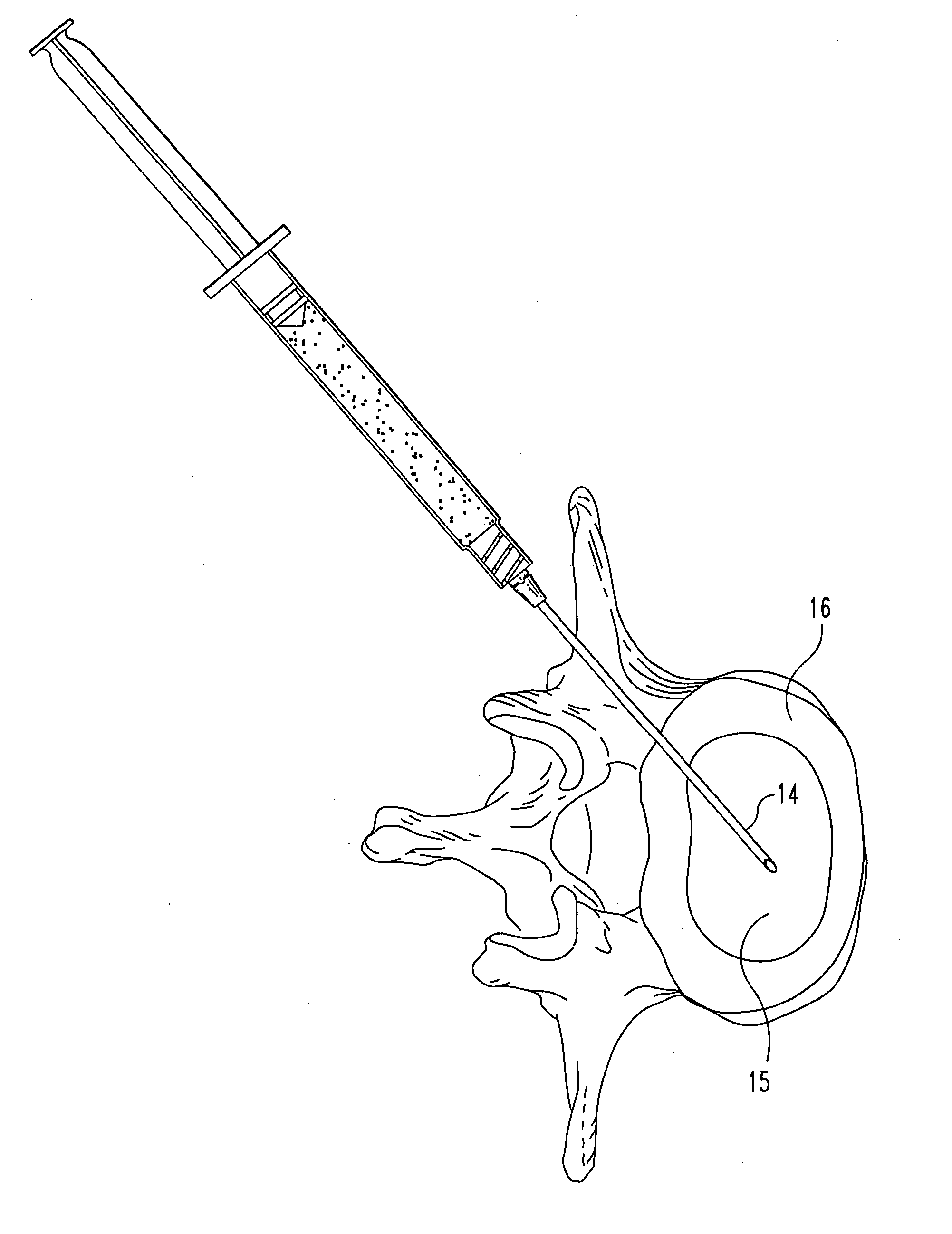
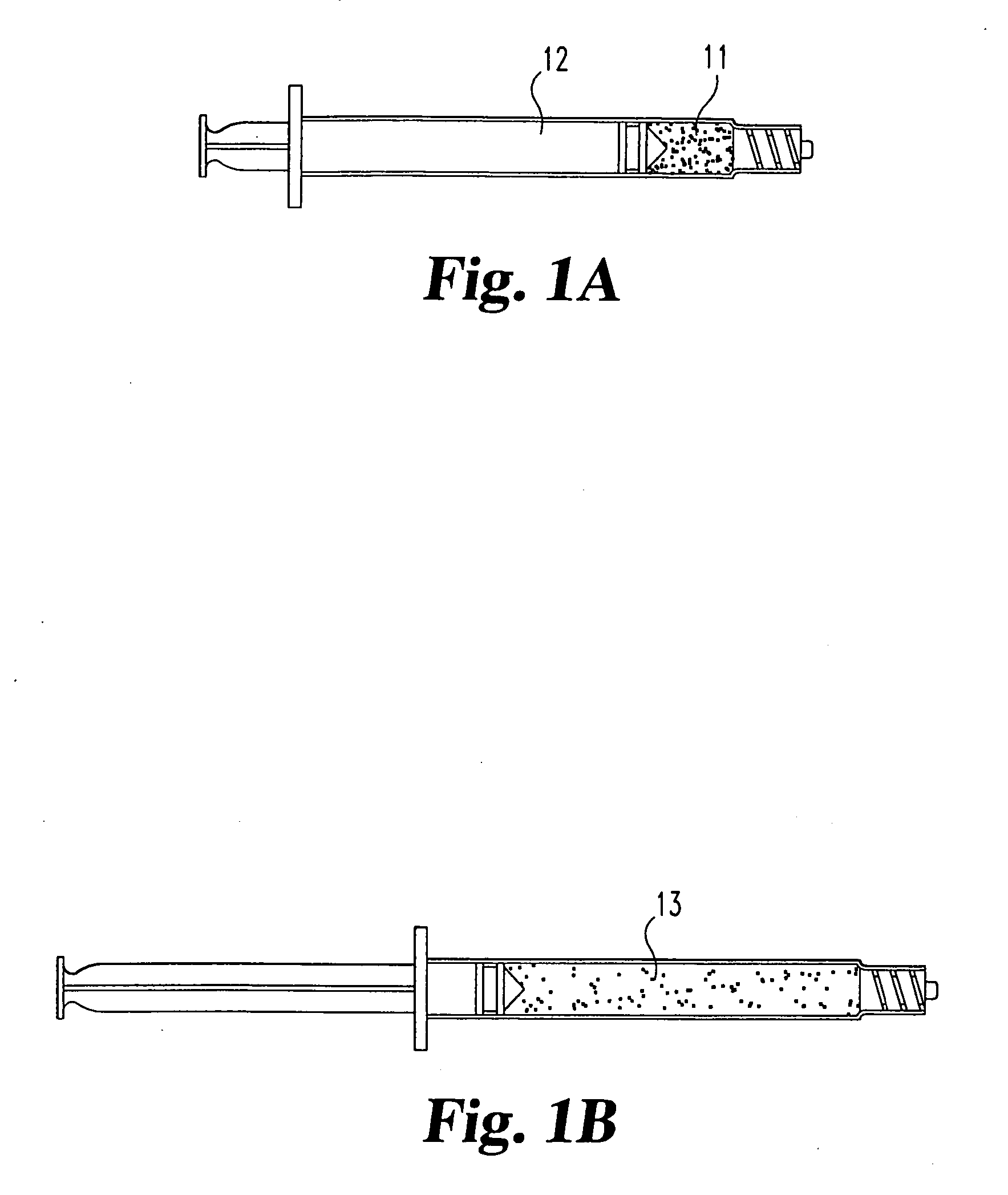
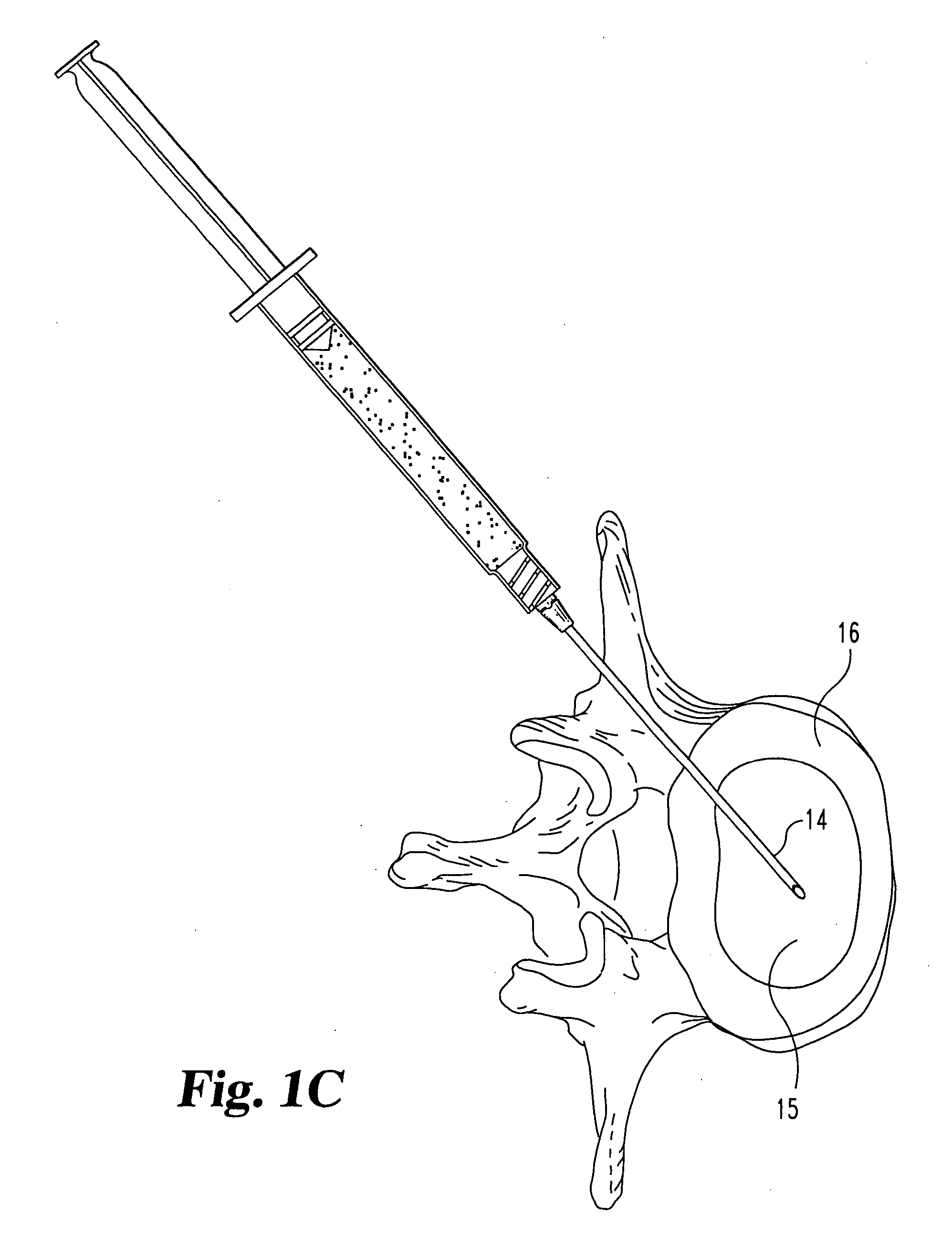
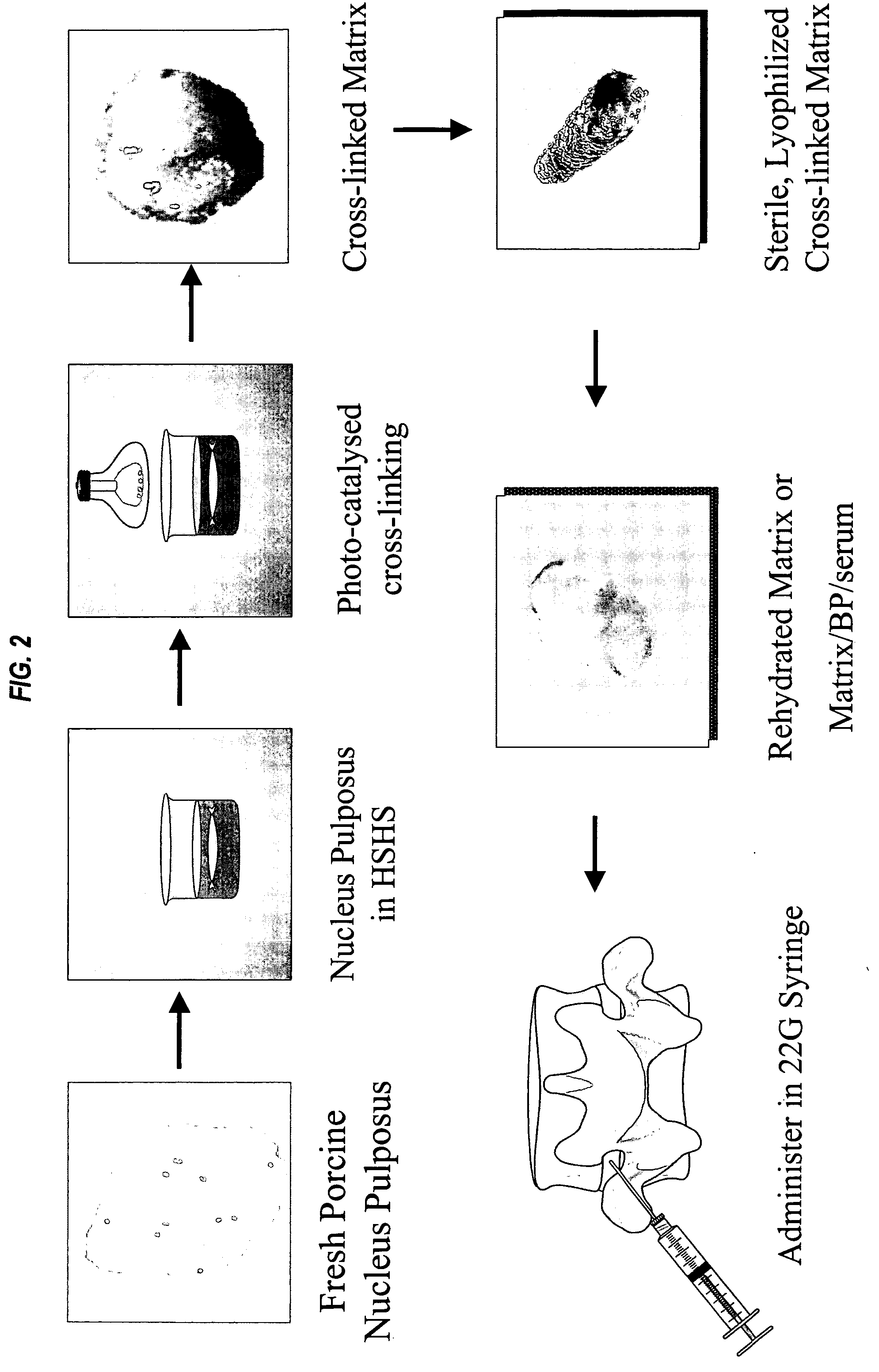
Compositions and methods for treating intervertebral discs with collagen-based materials
ActiveUS20050119754A1Enhance the imagePromote healingInternal osteosythesisLigamentsIntervertebral discInjected material
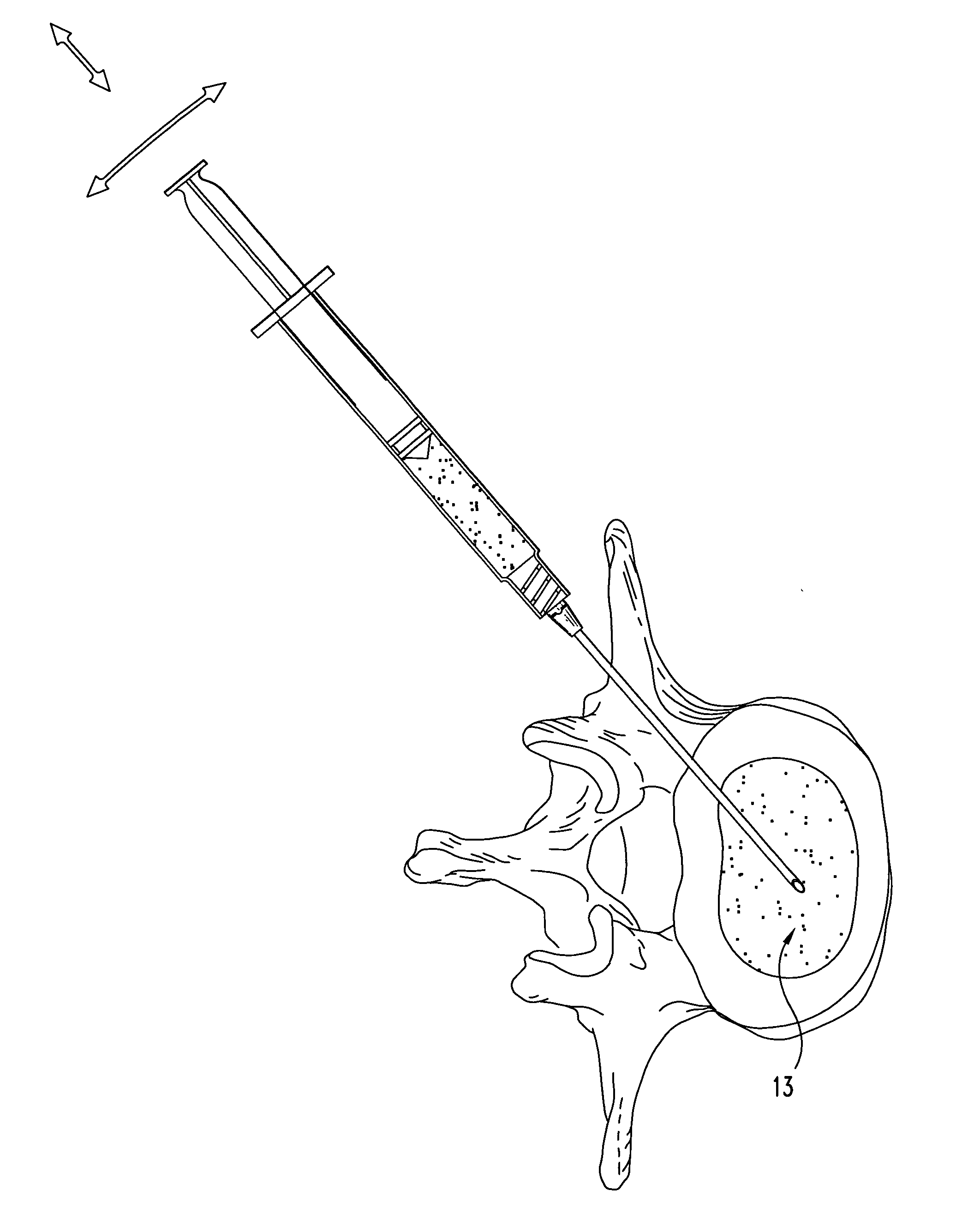
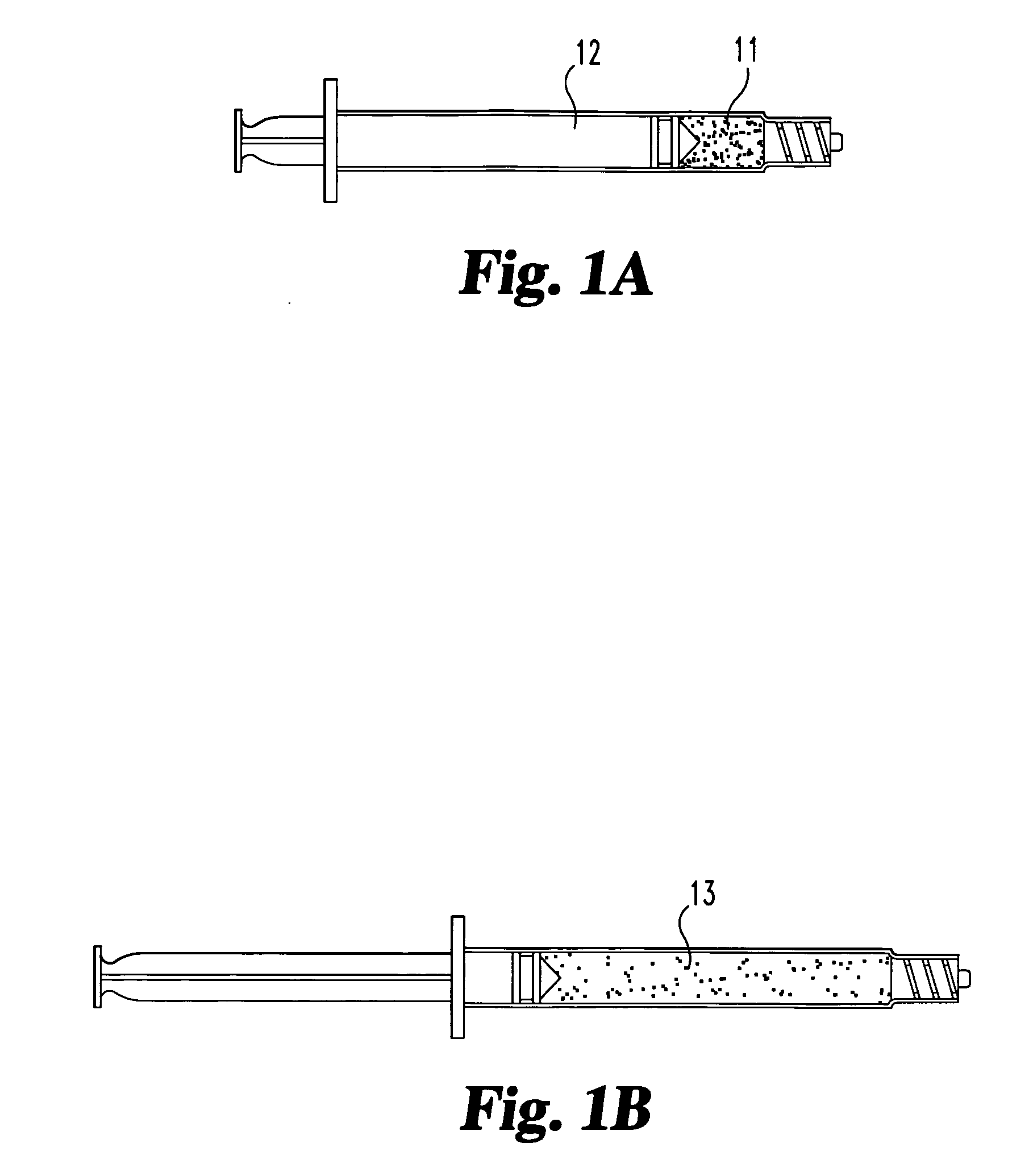
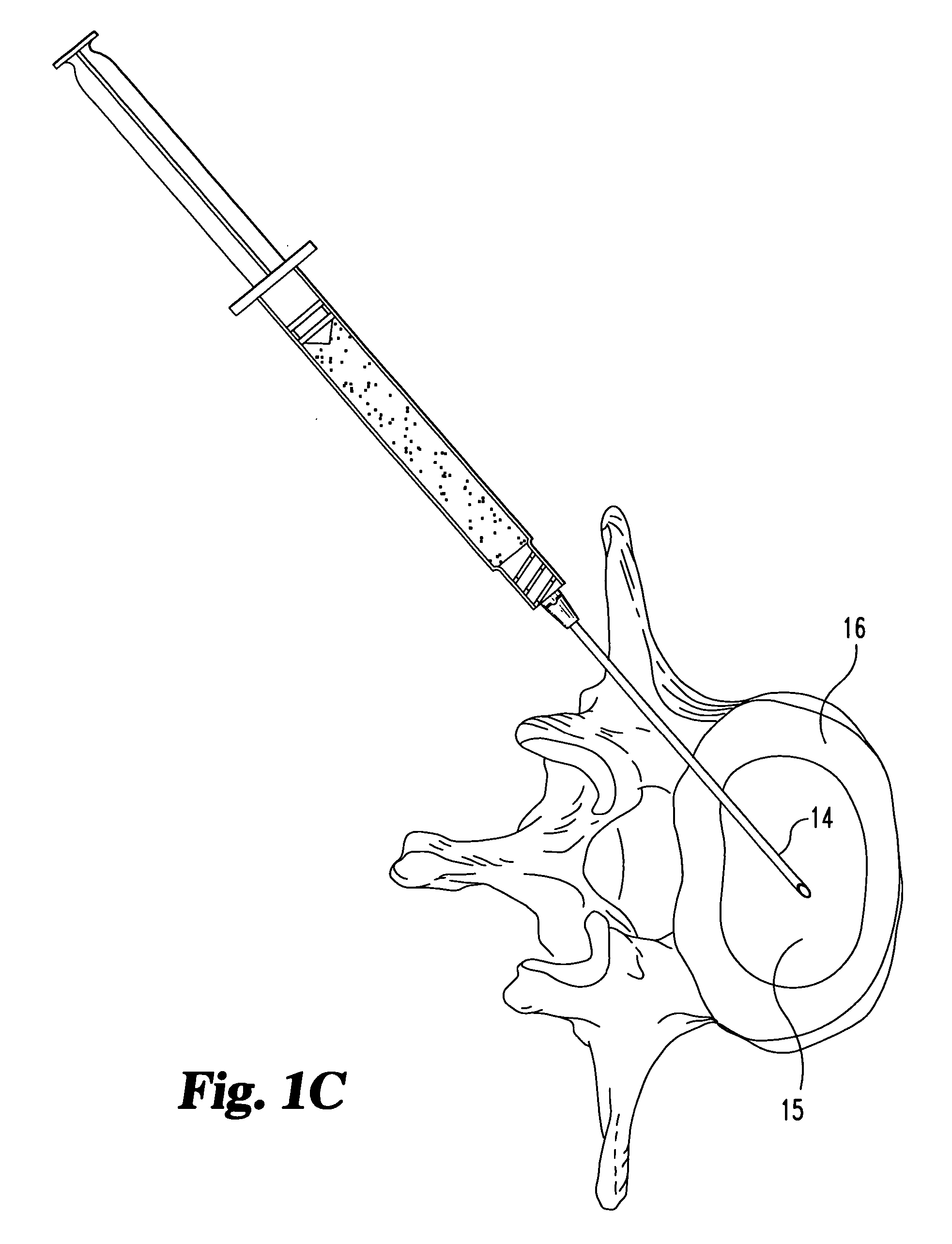
A method of augmenting an intervertebral disc nucleus by injecting or otherwise adding to a disc nucleus a plurality of particles of natural, collagen-rich tissue. The mean particle size of the pieces of natural, collagen-rich tissue may be between 0.25 mm and 1.0 mm. The particles may be dehydrated before implantation, and rehydrated after implantation, or they may be implanted in a “wet” state—such as a slurry or gel. Radiocontrast materials may be included to enhance imaging of the injected material. Other additives may include analgesics, antibiotics, proteoglycans, growth factors, stem cells, and / or other cells effective to promote healing and / or proper disc function.
Owner:WARSAW ORTHOPEDIC INC
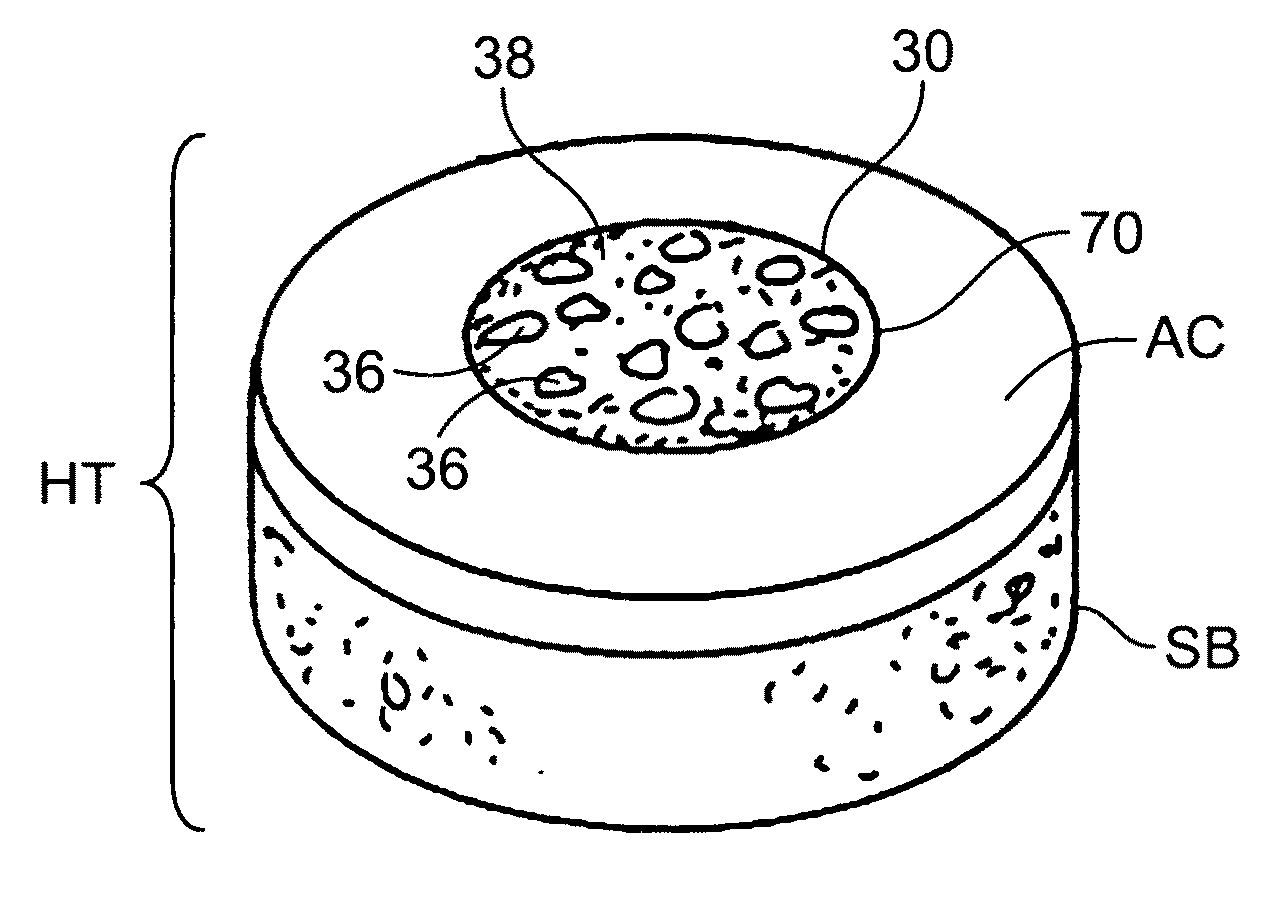
Cartilage implant assembly and method for implantation
InactiveUS20050222687A1Recovery functionRelief the painBone implantSurgerySubchondral boneInterference fit
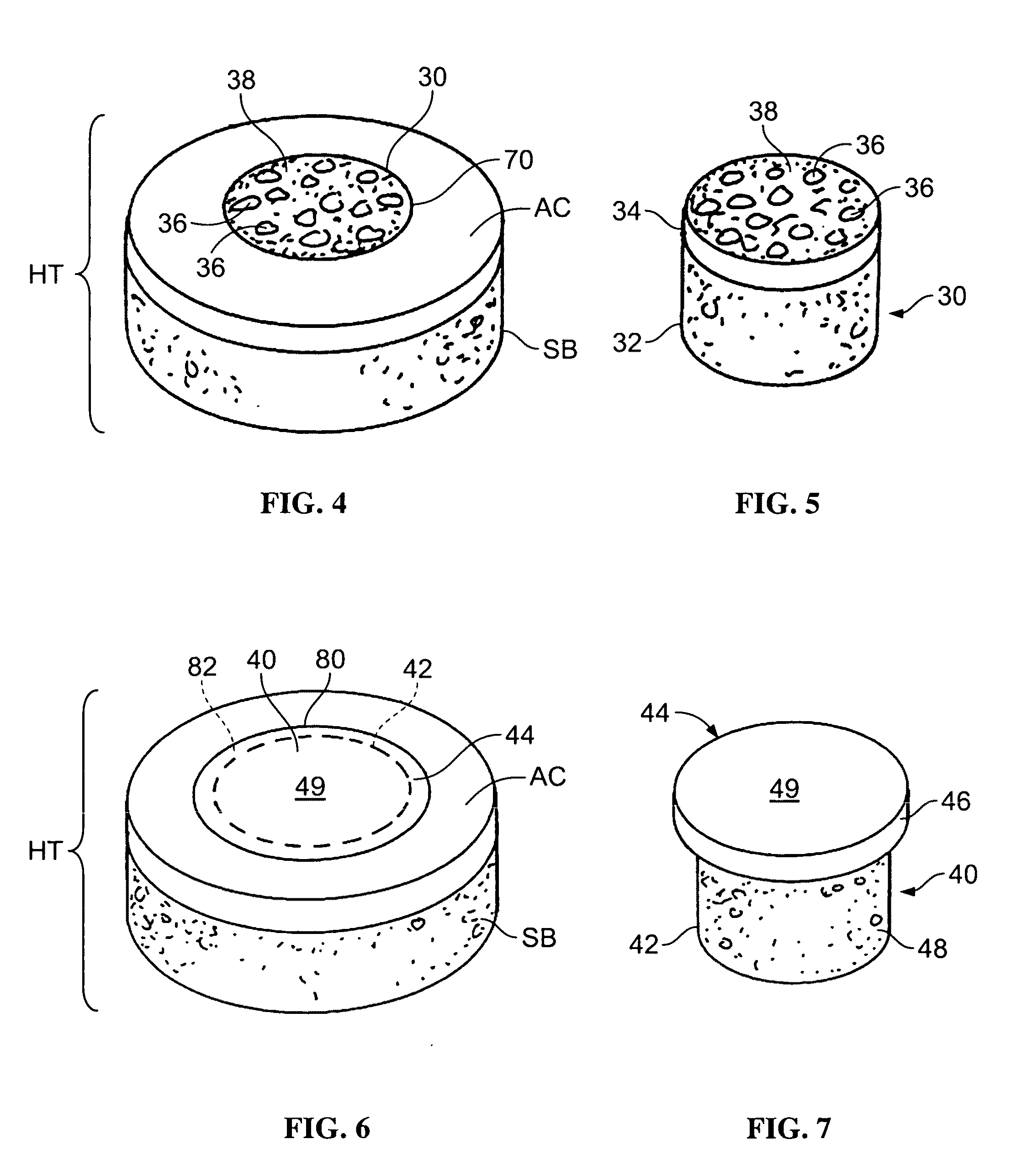
The invention is directed toward a cartilage repair assembly comprising a shaped structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that said shaped bone and cartilage cap when centered in the bore does not engage the side wall of the bore in an interference fit and is surrounded by milled cartilage and carrier. A method for inserting the assembly into a cartilage defect area is disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC +1
Cartilage allograft plug
InactiveUS20050251268A1Increase chondrocyte migrationIncreased proliferationBone implantLigamentsInterference fitSubchondral bone
The invention is directed toward a cartilage repair assembly comprising a cylindrically shaped allograft structure of subchondral bone with an integral overlying smaller diameter cartilage cap which is treated to remove cellular debris and proteoglycans. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the subchondral bone of the structure engages the side wall of the bone portion of the drilled bore in an interference fit while the cartilage cap is spaced from cartilage portion of the side wall of the drilled bore forming a gap in which a milled cartilage and biocompatible carrier mixture is placed allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Stem cells within gel microenvironments
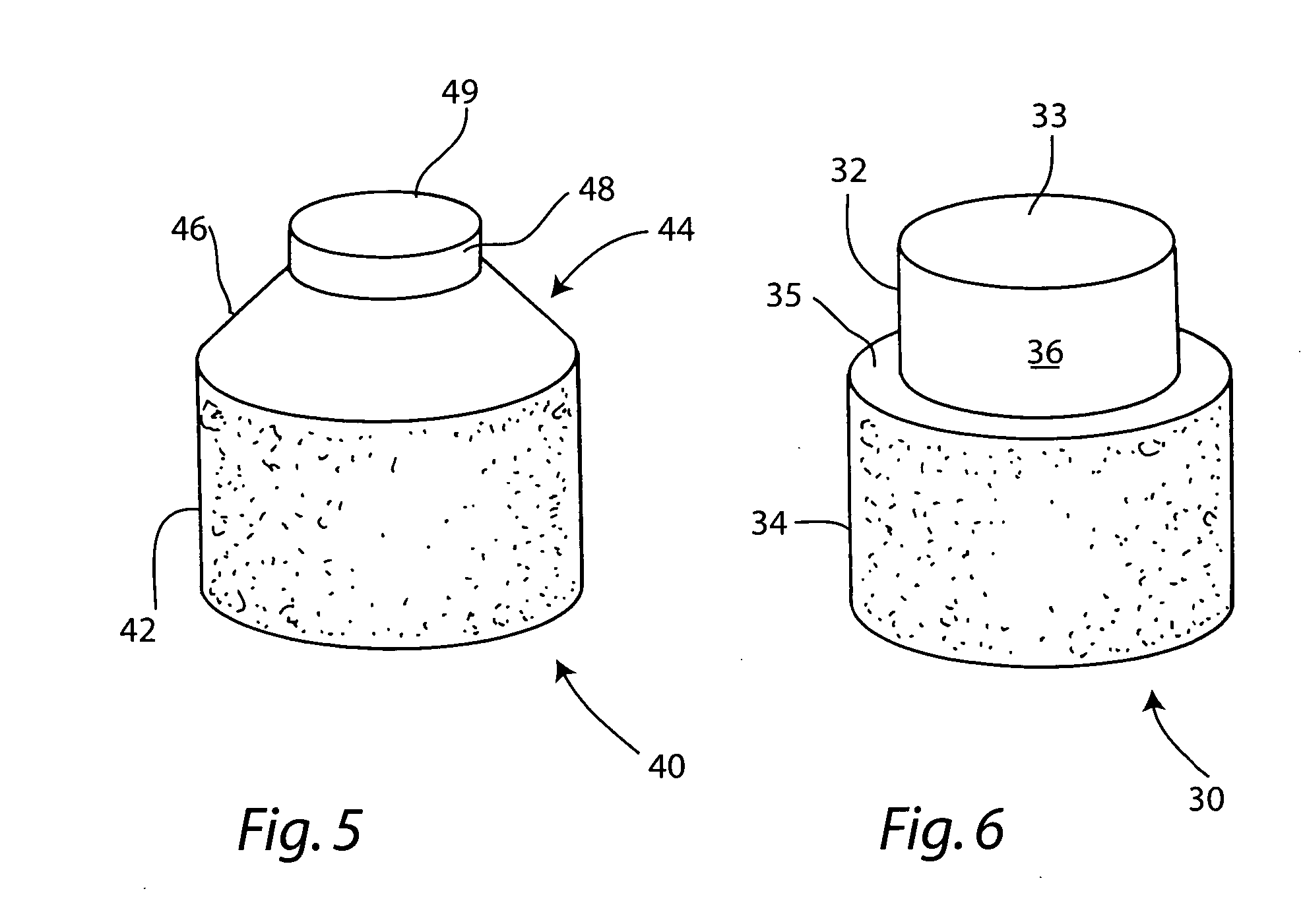
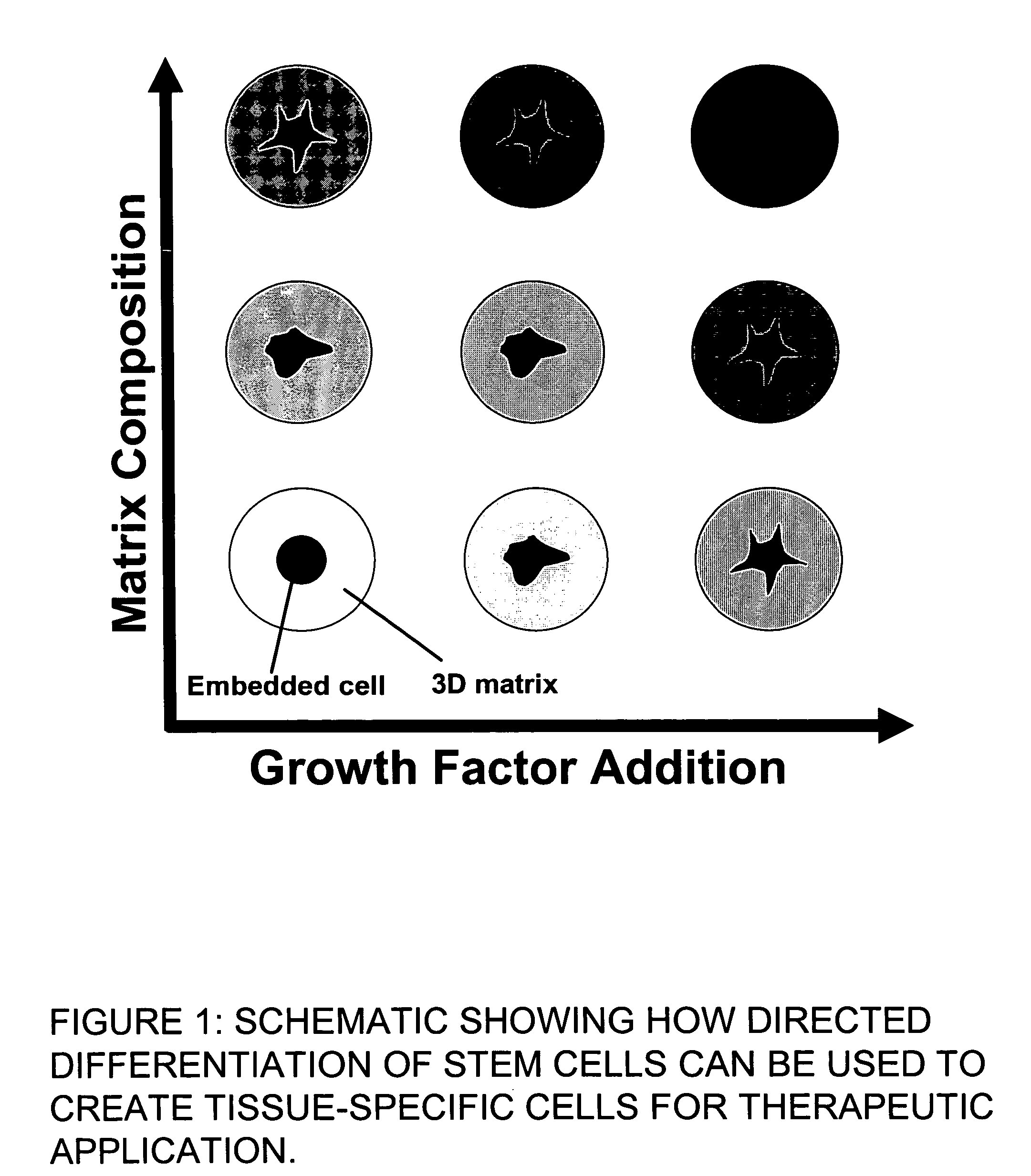
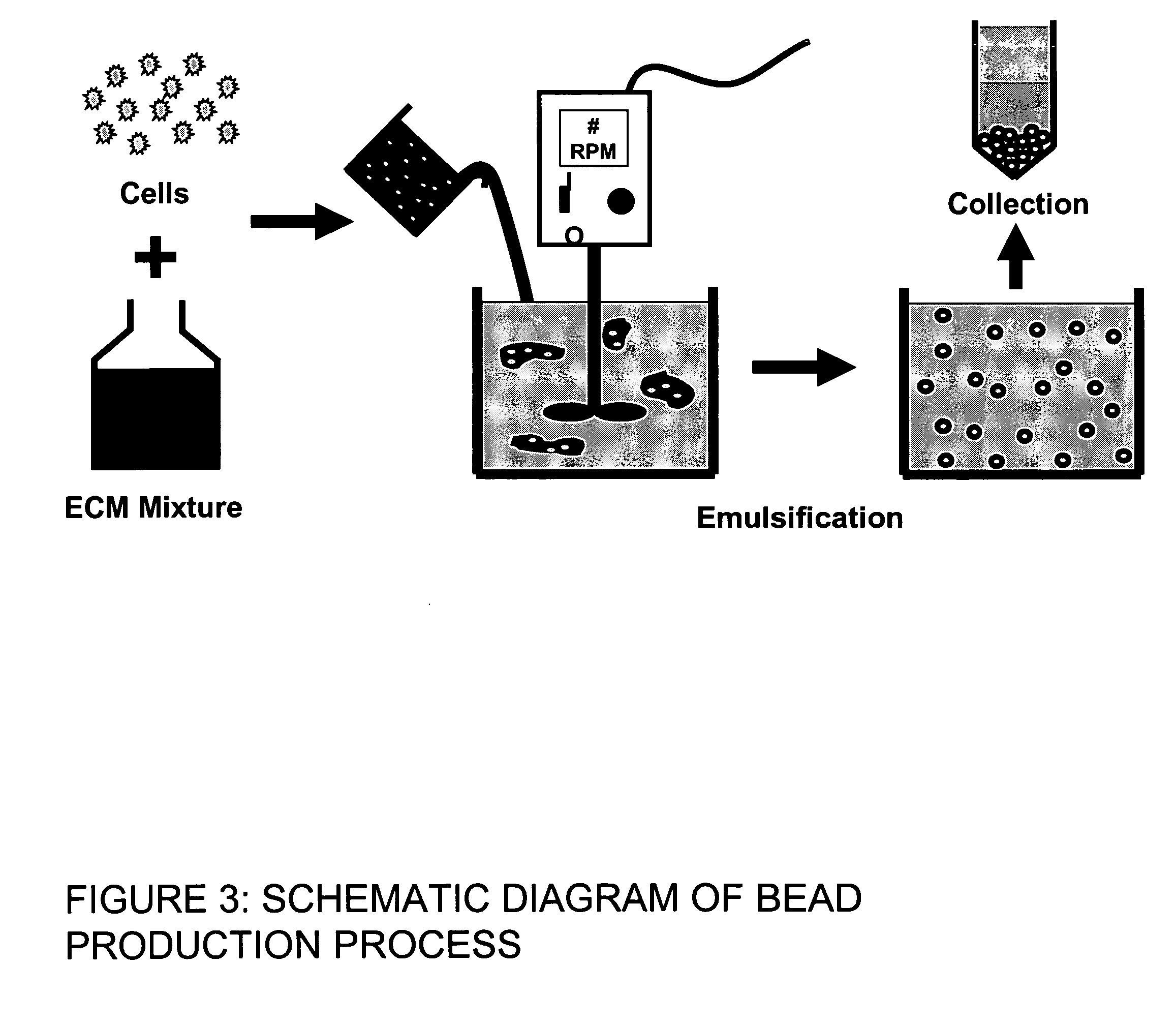
This invention provides a system to embed stem cells within three-dimensional (3D) hydrogel microenvironments consisting of naturally derived proteins, proteoglycans and / or polysaccharides. Pure matrices or combinations of materials can be used. The method involves suspending stem cells in solutions of the matrix components of interest, emulsifying these solutions in a hydrophobic phase, triggering gelation of the matrix components by changing the environmental conditions, and collection of the resulting hydrogel beads. The unique bead format of this invention has the advantage of allowing the use of small amounts of rare matrix proteins. Bead preparations can be concentrated into a paste for use as a cell delivery vehicle to damaged tissues, either directly after encapsulation or after a period of culture to promote stem cell differentiation. Defined 3D microenvironments can guide stem cell differentiation, and the resulting beads can be used directly as a cell delivery vehicle in various tissue repair applications.
Owner:RENESSELAER POLYTECHNIC INST
Collagen-based materials and methods for augmenting intervertebral discs
ActiveUS20050197707A1Enhance the imagePromote collagen crosslinkingInternal osteosythesisPeptide/protein ingredientsIntervertebral discInjected material
A method of augmenting an intervertebral disc by injecting particles of collagen-based material into the disc. The particles may be dehydrated before implantation, and rehydrated after implantation, or they may be implanted in a “wet” state—such as a slurry or gel. Radiocontrast materials may be included to enhance imaging of the injected material. Other additives may include analgesics, antibiotics, proteoglycans, growth factors, and / or other cells effective to promote healing and / or proper disc function.
Owner:WARSAW ORTHOPEDIC INC
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with HER-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080176258A1Improve efficiencyIncrease resourcesBiological material analysisDepsipeptidesImmunodominant EpitopesMonoclonal antibody
Disclosed herein among other MN / CA IX-related inventions are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Subsets of the new antibodies are to either the proteoglycan-like (PG) domain or to the carbonic anhydrase (CA) domain of MN / CA IX, and methods are provided by which antibodies can be prepared to the other MN / CA IX domains. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Method of treating mast cell activation-induced diseases with a proteoglycan
InactiveUS6689748B1Decrease in urinaryBiocidePeptide/protein ingredientsInflammatory Bowel DiseasesInterstitial cystitis
The invention provides a method for preventing and treating the harmful biological effects of biochemicals secreted from activated mast cells in the organism of warm blooded animals and more especially human beings, said effects being associated with allergy (including but not limited to allergic conjunctivitis, allergic rhinitis, allergic otitis, asthma, allergic uticaria, food allergy and atopic dermatitis), hyperproliferative diseases such as leukemia and systemic mastocytosis, interstitial cystitis, inflammatory bowel disease, irritable bowel syndrome, osteoporosis and scleroderma. The method consists in administering to said animals and especially to human beings an effective amount of a proteoglycan such as chondroitin sulfate with mast cell secretion inhibitory activity, alone or in combination with one or more synergistic adjuvants such those belonging to the class of flavonoids or compounds with histamine-1 receptor antagonist activity.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
Allograft osteochondral plug combined with cartilage particle mixture
InactiveUS20090291112A1Promoting cartilage cell migration intoPromoting proliferationBiocidePeptide/protein ingredientsInterference fitSubchondral bone
An allograft osteochondral plug is combined with a mixture that includes freeze-milled cartilage particles, and such combination is used to repair defects in articular cartilage. The plug includes an subchondral bone portion and an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans. At least a portion of the plug has a lateral dimension selected to form an interference fit against a tissue layer exposed as a result of a bore formed in a defect area in articular cartilage of a host. The cartilage particle mixture is placed adjacent at least a portion of the plug for promoting cartilage cell migration into (i.e., from the adjacent host cartilage) and proliferation in the bore, and for enhancing tissue integration between the plug and patient (i.e., host) tissue when the plug is inserted into the bore. Methods for surgical implantation of the plug into a patient are also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Tissue transplantation compositions and methods
A biomedical material for transplant to a subject is provided according to embodiments of the present invention which includes an isolated donor tissue enzyme-treated to reduce the amount of proteoglycans in the donor tissue compared to untreated tissue. Isolated cells are optionally added to the enzyme-treated donor tissue, including leukocytes, particularly monocytes; macrophages; platelets; cells derived from an intervertebral disc such as chondrocyte-like nucleus pulposus cells; fibrocytes; fibroblasts; mesenchymal stem cells; mesenchymal precursor cells; chondrocytes; or a combination of any of these. The isolated donor tissue is articular cartilage or an intervertebral disc tissue such as nucleus pulposus tissue and / or annulus fibrosis tissue enzyme-treated to remove proteoglycans normally present in these tissues. A biomedical material of the present invention is administered to a subject to treat a disorder or injury, such as a disorder or injury to connective tissue.
Owner:FERREE BRET
C-glycoside compounds for stimulating the synthesis of glycosaminoglycans
InactiveUS7049300B2Increase synthesisFunction increaseBiocideCosmetic preparationsC-glycosideN Acetyl D Glucosamine
C-glycoside compounds are suited for stimulating the synthesis of glycosaminoglycans containing a D-glucosamine and / or N-acetyl-D-glucosamine residue, advantageously hyaluronic acid, and / or proteoglycans, advantageously proteoglycans containing hyaluronic acid, by fibroblasts and / or keratinocytes.
Owner:LOREAL SA
Cosmetic compositions
A cosmetic composition comprising in a preblend; or in a the resting state prior to application to a keratinous surface, at least one peptide, at least one collagen containing compound, at least one penetration enhancer, at least one mucopolysaccharide, and at least one proteoglycan; wherein said ingredients are operable to associate in situ when the composition is applied to a keratinous surface and a method for plumping lips or skin by applying the composition.
Owner:REVLON CONSUMER PROD CORP
Composition for protection against superficial vasodilator flush syndrome
InactiveUS20050220909A1Promote absorptionBiocideCosmetic preparationsChondroitin Sulfate CVascular dilatation
Compositions with synergistic anti-inflammatory effects in inflammatory diseases resulting from activation and consequent degranulation of mast cells and followed by secretion of inflammatory biomolecules from the activated mast cells, composed of a heavily sulfated, non-bovine proteoglycan such as shark cartilage chondroitin sulfate C, an unrefined olive kernel oil / extract that increases absorption of these compositions in various routes of administration, and one or more of a hexosamine sulfate such as D-glucosamine sulfate, a flavone such as quercetin, S-adenosylmethionine, a histamine-1 receptor antagonist, a histamine-3 receptor agonist, an antagonist of the actions of CRH, caffeine, and a polyamine.
Owner:THEOHARIDES THEOHARIS C
Methods of promoting healing of cartilage defects and method of causing stem cells to differentiate by the articular chondrocyte pathway
InactiveUS20060111778A1Promote healingBone implantSkeletal/connective tissue cellsCell-Extracellular MatrixArticular chondrocyte
Methods of promoting healing of a cartilage defect in a region of cartilage, which comprises the defect and which may further comprise stem cells, and methods of promoting healing of a cartilage defect in a region of cartilage, which comprises the defect and an implant comprising cartilage scaffold or a cartilage graft, which methods comprise contacting the region with various combinations of cartilage fragments, a growth factor, a partially synthesized extracellular matrix, a scaffold, an implant comprising cartilage scaffold, an implant comprising a cartilage graft, stem cells, chondrocytes, a proteoglycan, an anti-oxidant, a collagen precursor, a vitamin, a mineral, and / or a cartilage-degrading enzyme; and a method of causing stem cells to differentiate by the articular chondrocyte pathway comprising contacting the stem cells with a compound comprising an active alcohol moiety.
Owner:MICHALOW ALEXANDER E
Compositions and Methods for Treating a Disorder or Defect in Soft Tissue
The present invention encompasses methods and compositions for generating a biomimetic proteoglycan. The invention includes methods of treating a disease, disorder, or condition of soft tissue using a biomimetic proteoglycan.
Owner:DREXEL UNIV
Cartilage implant plug with fibrin glue and method for implantation
InactiveUS20080274157A1Guaranteed functionEasy to placeSurgical adhesivesPeptide/protein ingredientsSubchondral boneFibrin glue
The invention is directed toward a cartilage repair assembly comprising a shaped structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that said shaped bone and cartilage cap when centered in the bore does not engage the side wall of the bore and is positioned from the side wall of the bone a distance ranging from 10 microns to 1000 microns and is surrounded by milled cartilage and a fibrin thrombin glue. A method for inserting the assembly into a cartilage defect area is disclosed.
Owner:MASSACHUSETTS INST OF TECH +1
Methods and compositions for treating intervertebral disc degeneration
A fluid matrix comprising cross-linked remodelable collagen from a donor vertebrate animal is useful for regenerating hydrodynamic function in damaged intervertebral discs in vivo. The matrix may be injectable and may comprise cells and a plurality of purified cell growth factors. The matrix promotes cell growth and elaboration of proteoglycans to facilitate regeneration of native tissues. The collagen in the matrix may be cross-linked using photooxidative catalysis and visible light, and purified cell growth factors are preferably at least partly bone-derived.
Owner:ZIMMER ORTHOBIOLOGICS
Novel C-glycosides, uses thereof
The present invention relates to novel C-glycoside compounds of given absolute configuration, to a process for synthesising them and to compositions containing them. The invention also relates to the cosmetic use of these C-glycoside compounds as agents to stimulate the synthesis of glycosaminoglycans containing a D-glucosamine and / or N-acetyl-D-glucosamine residue, advantageously hyaluronic acid, and / or of proteoglycans, advantageously proteoglycans containing hyaluronic acid, by fibroblasts and / or keratinocytes. The invention also relates to a cosmetic process for treating keratin materials using a composition containing at least one C-glycoside compound according to the invention.
Owner:LOREAL SA
Compositions for protection against superficial vasodilator flush syndrome, and methods of use
ActiveUS20070141187A1Promote absorptionBiocideCosmetic preparationsSulfate proteoglycanVascular dilatation
Compositions for protection against SVFS syndromes are composed of a flavonoid compound of the basic structures 2-phenyl-4H-1-benzopyran or 2-phenyl-4-keto-1-benzopyran or glycosides thereof, an inventive olive kernel extract and a non-bovine sulfated proteoglycan, and, optionally, one or more of bitter willow extract, D-glucosamine sulfate and serotonin inhibitor.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
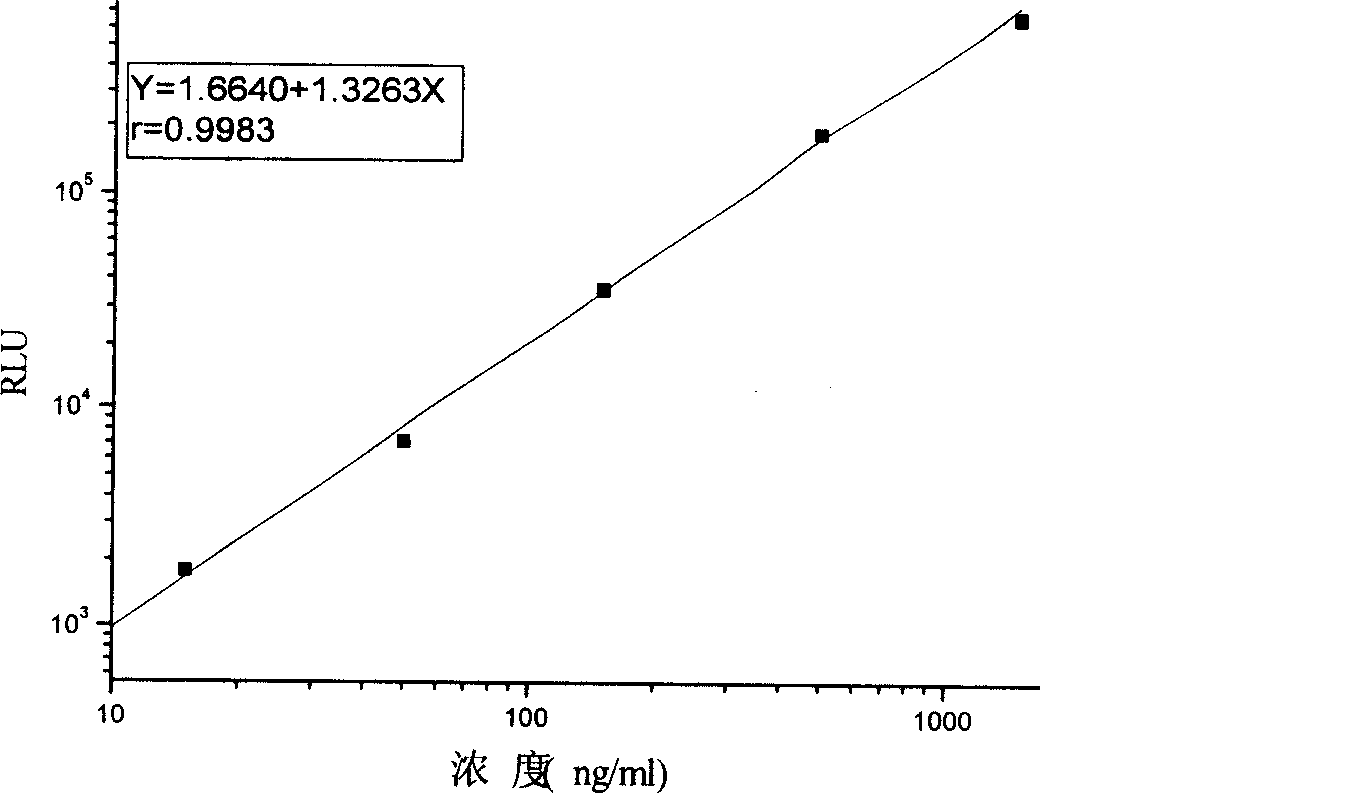
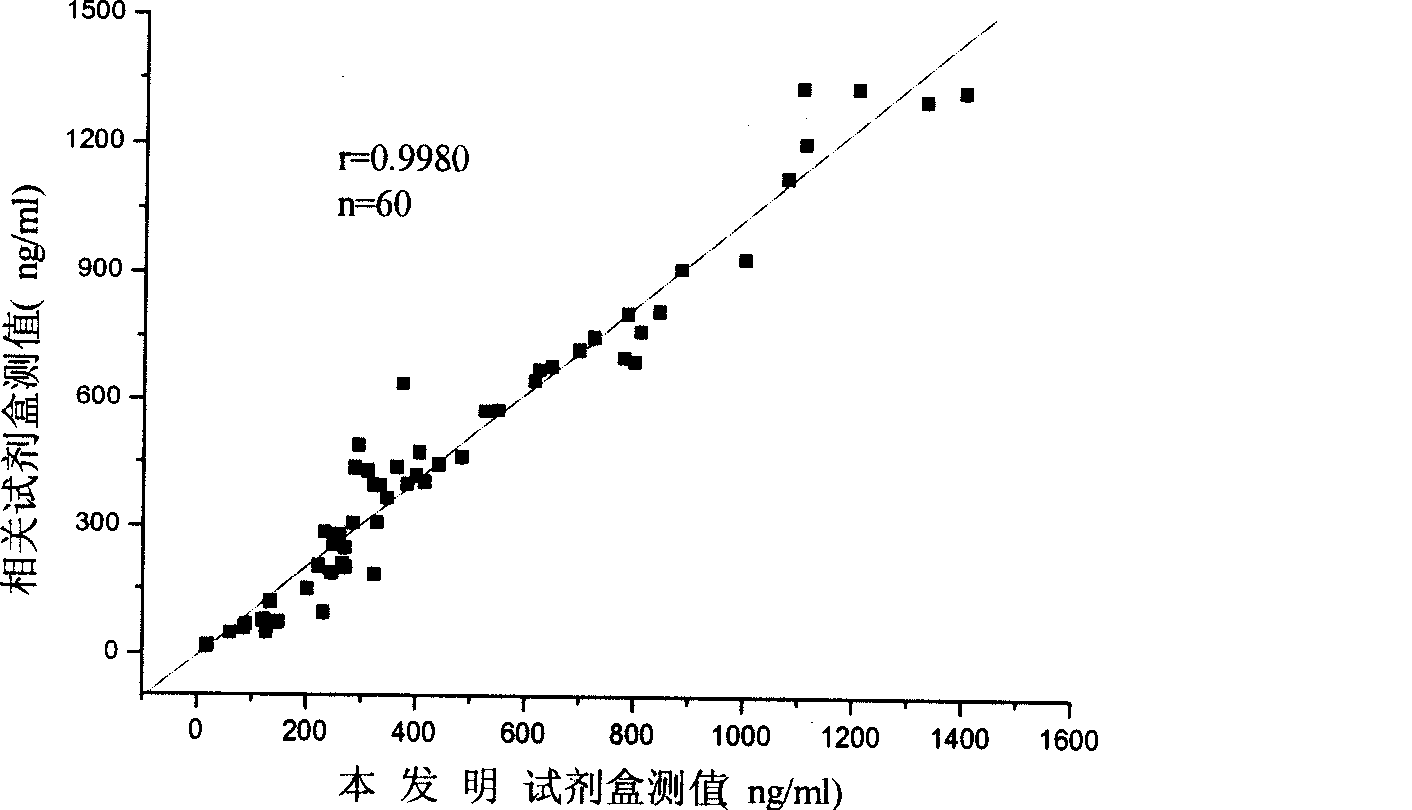
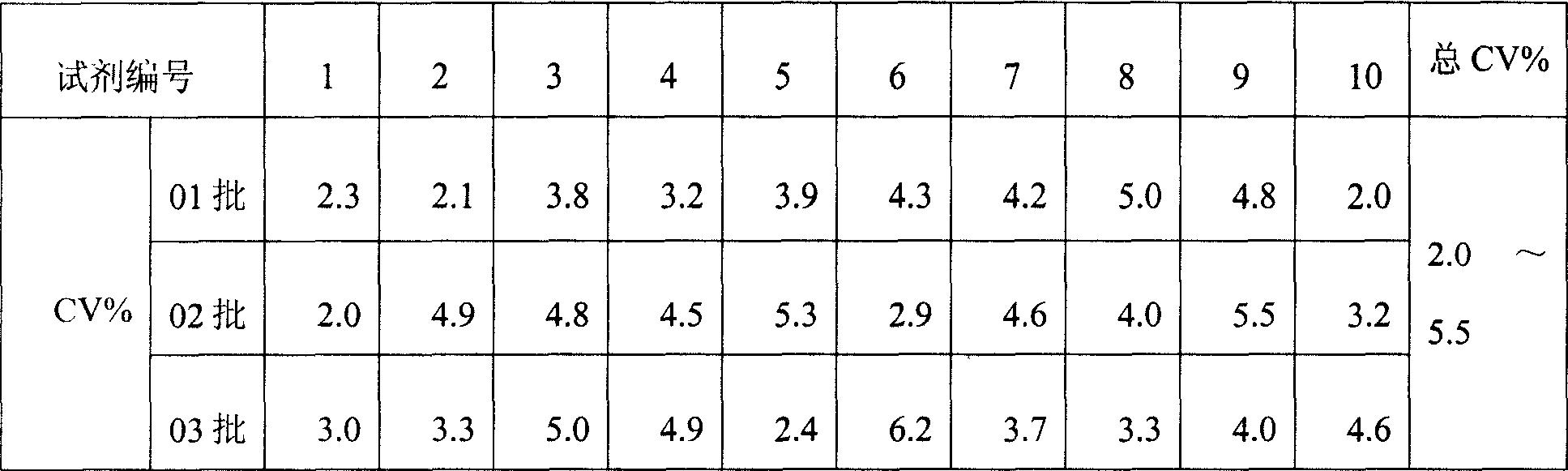
Monophosphoinositide proteoglycans-3 chemiluminescence immune analysis determination reagent kit and preparing method thereof
InactiveCN101377506AIncrease the effective amountReliable clinical reference valueChemiluminescene/bioluminescenceTreatment effectChemiluminescent immunoassay
The invention relates to the medical field of immunoassay, more specially, the invention provides a chemiluminescent immunoassay detection kit for phosphatidylinositol proteoglycan-3(GPC-3) and a preparation method thereof, and realizes the simultaneous serological detection of GPC-3 N terminal and C-terminal protein with the chemiluminescent immunoassay method. The kit has the advantages of simple sampling, convenient detection and accurate and specific technical method. The invention adopts a biotin-strapavidin system to coat antibodies and improve the efficiency of antibody coating and the linear range of detection as well as sensitivity, and can be conveniently used for the tracing observation of early diagnosis or treatment effect for primary carcinoma of liver.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
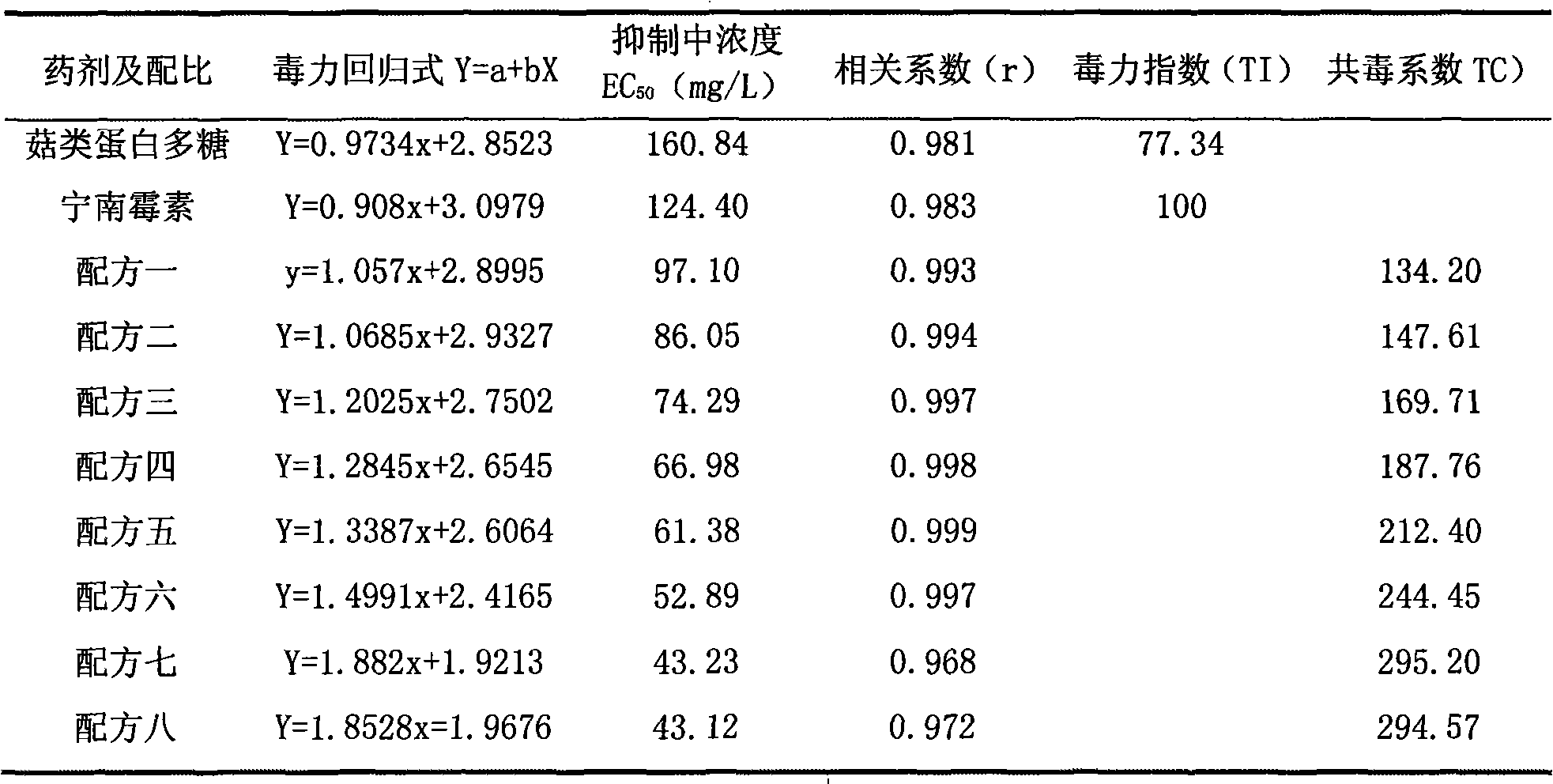
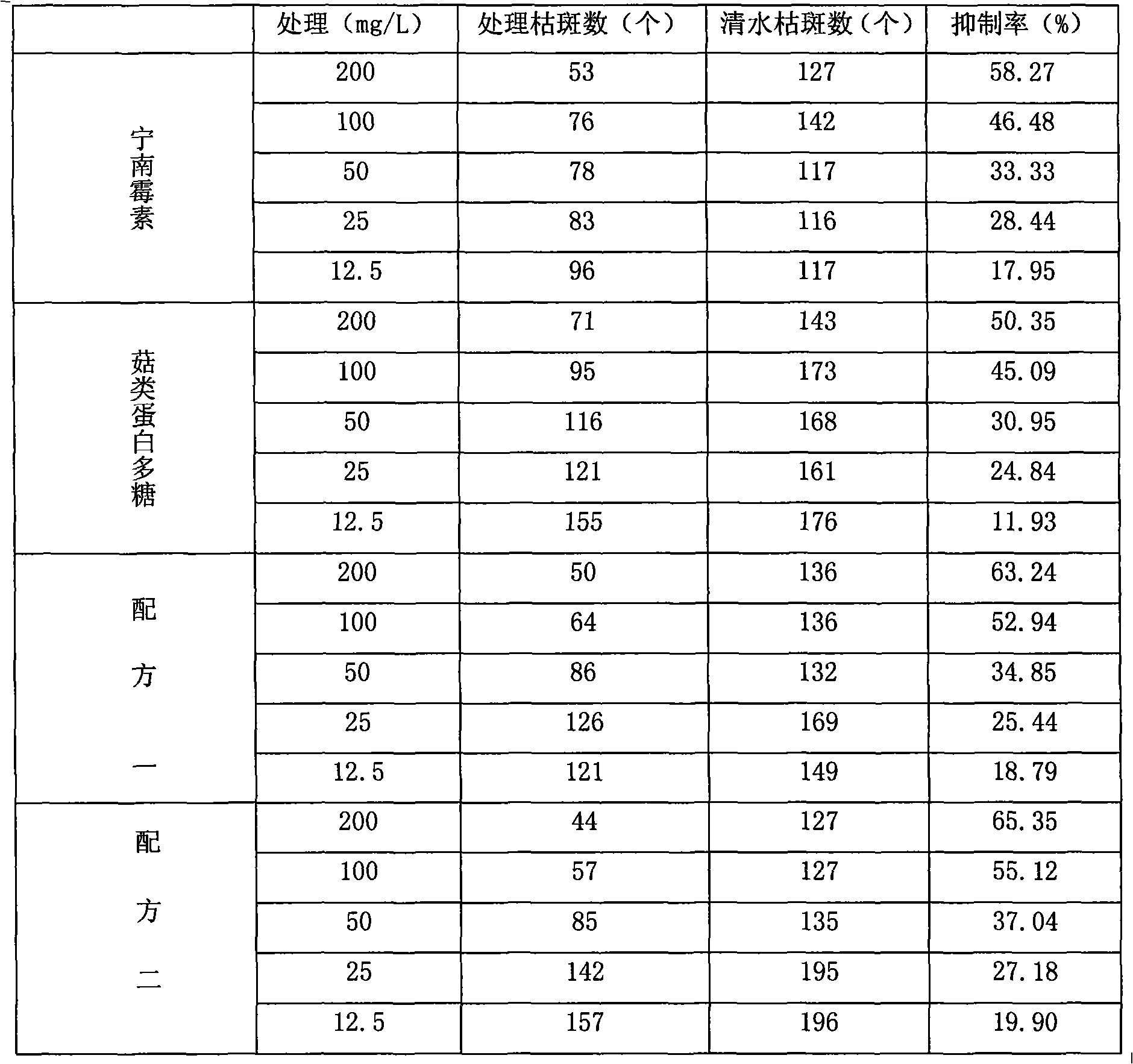
Bactericide agent composition containing mushroom proteoglycan
The invention belongs to the pesticide technical field, relates to a fungicide composite and is characterized in that the fungicide composite contains the following constituents by weight percentage: 0.1 to 20 percent of ningnanmycin and 0.1 to 10 percent of mushroom proteoglycan. The fungicide composite of the invention can be prepared into water aqua, suspending agent and wettable powder, is used for controlling virus diseases and has obvious effect of increasing yield and improving the quality of crops.
Owner:燕化永乐(乐亭)生物科技有限公司
Method for analyzing a glycomolecule
InactiveUS20050186645A1Simple sample preparationSimple processBiological material analysisBiological testingLipid formationStructure analysis
The invention relates generally the structural analysis of glycomolecule-containing macromolecules, such as those that occur either attached to proteins (proteoglycans, glycoproteins), lipids, or as free saccharides.
Owner:PROCOGNIA ISRAEL
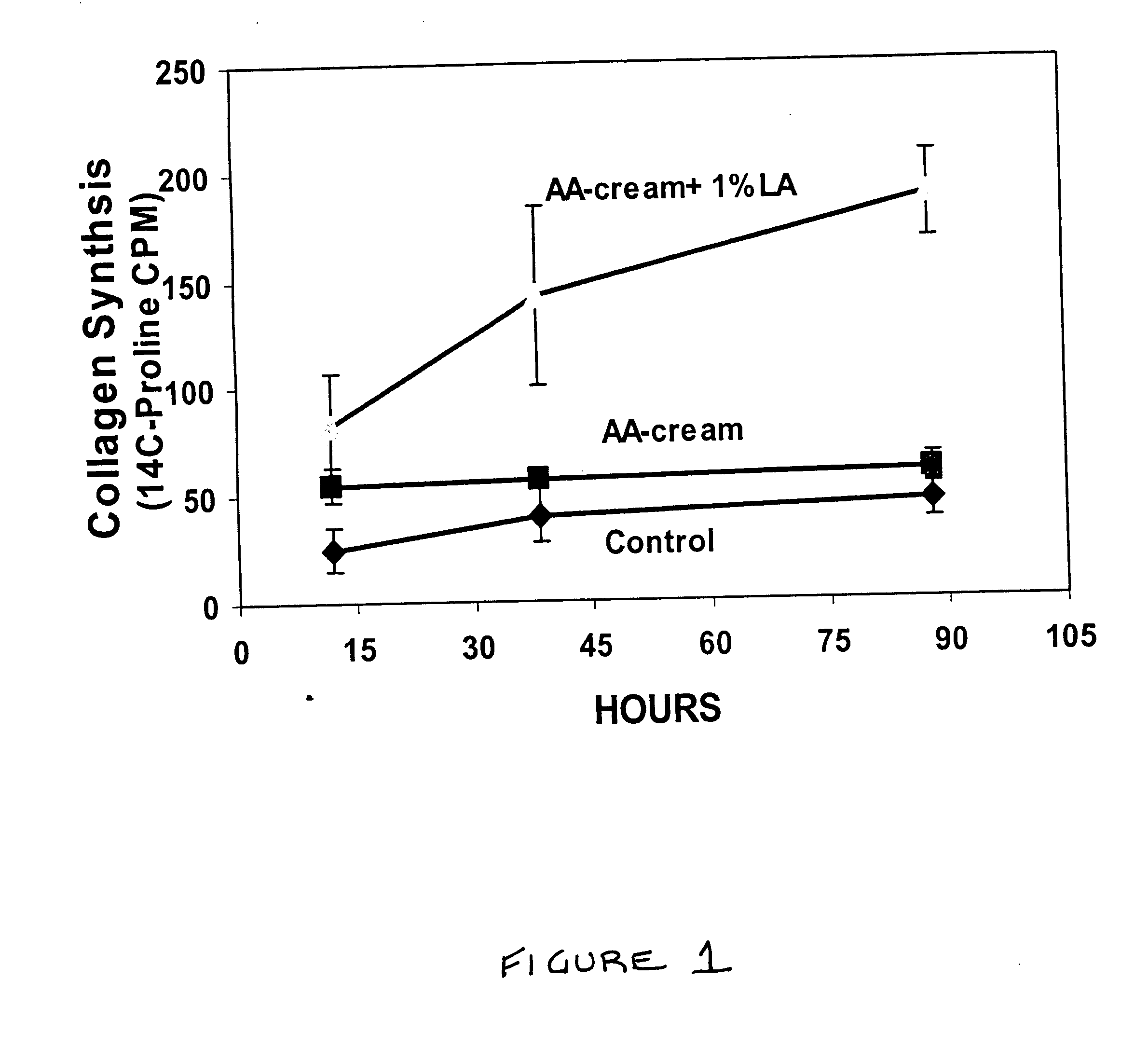
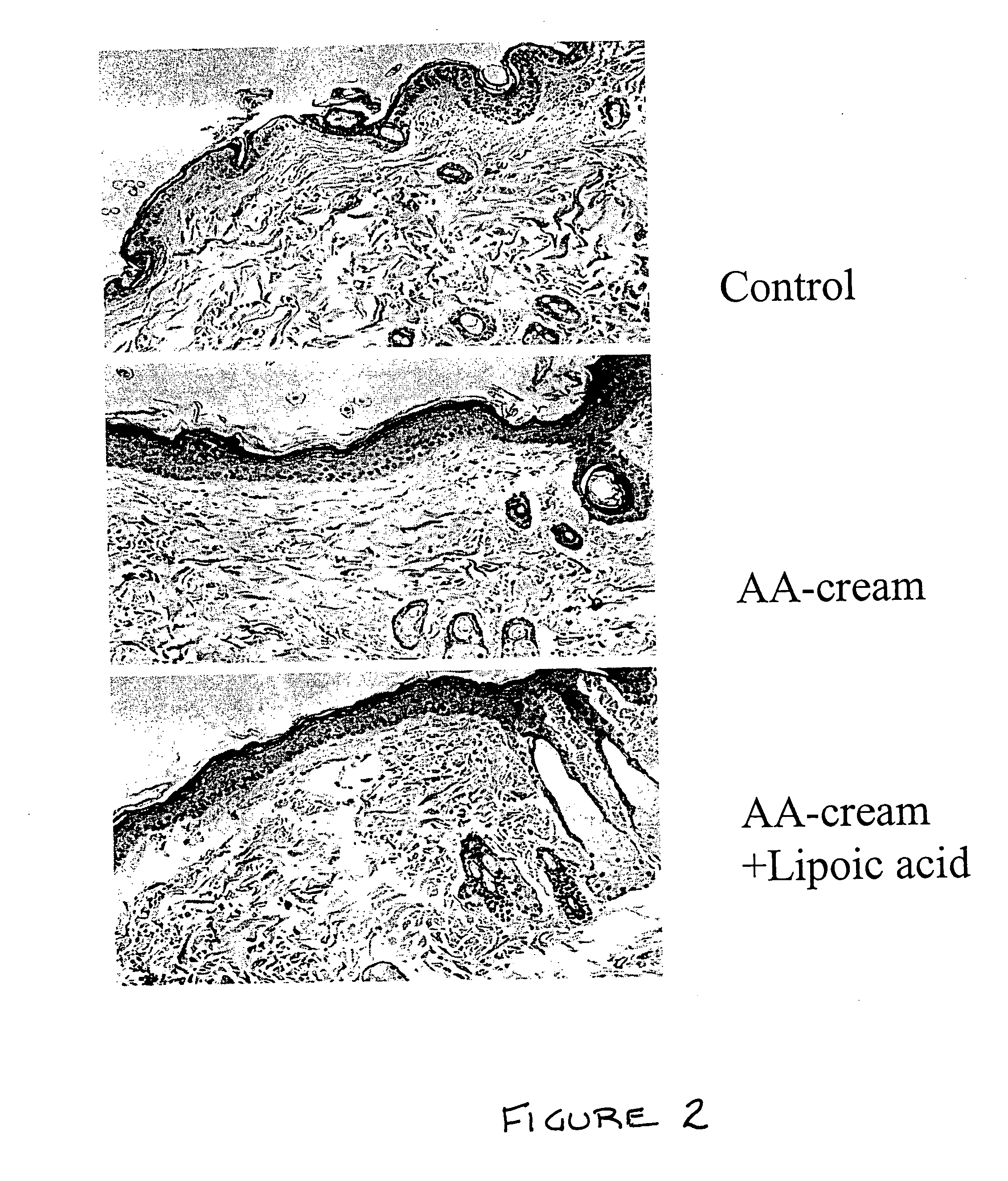
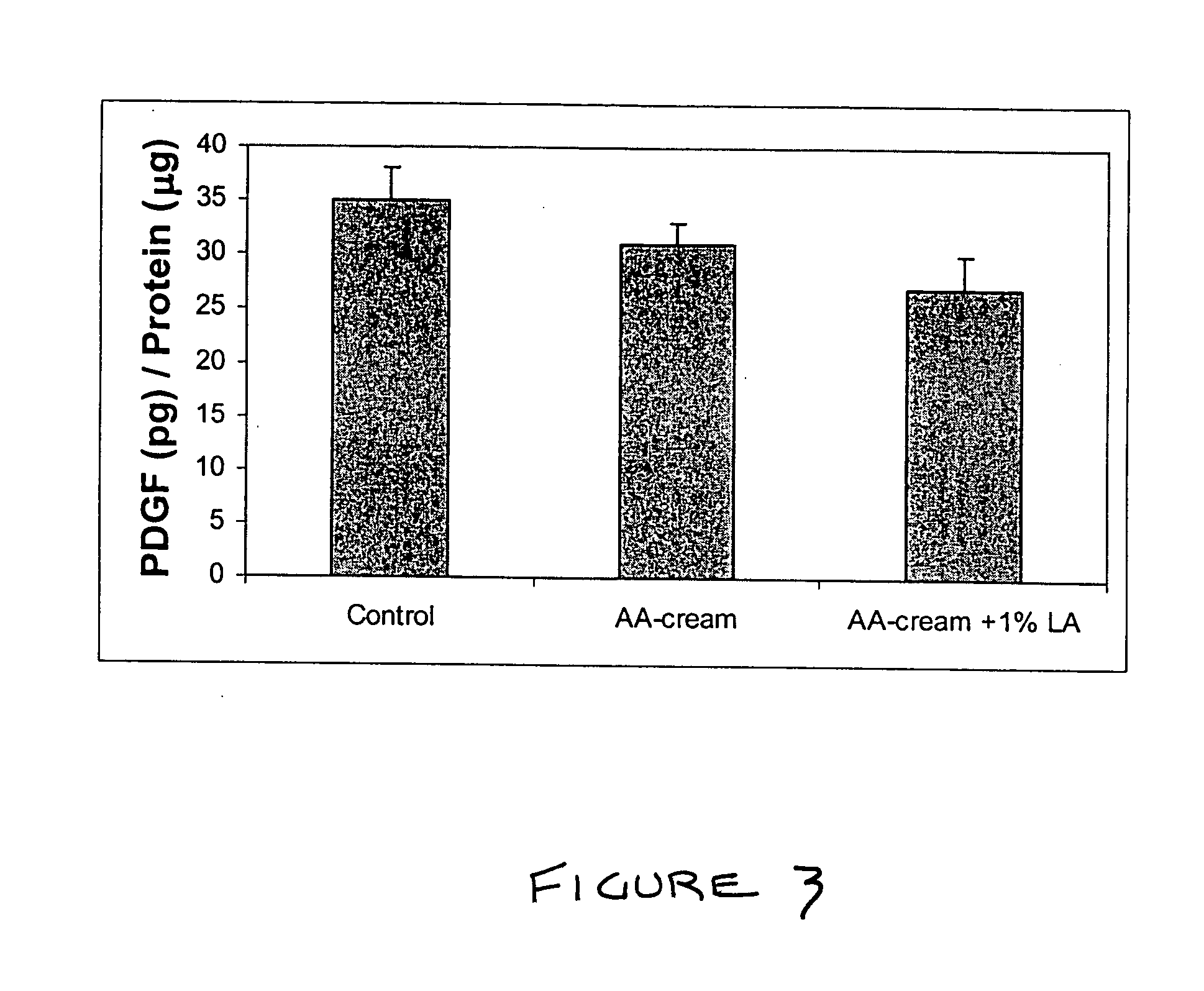
Methods and compositions for enhancing collagen and proteoglycan synthesis in the skin
ActiveUS20060045926A1Increase synthesisSustainable hydrationCosmetic preparationsBiocideAdditive ingredientIsoflavones
A composition for application to the skin can stimulate the in vivo synthesis of collagen and proteoglycans and improve the appearance of the skin, increasing its elasticity and fullness. In general, a composition according to the present invention comprises: (1) an antioxidant compound in a quantity sufficient to enhance collagen synthesis in the skin; (2) an organic penetrant in which the antioxidant compound is soluble in a sufficient quantity that a concentration of the antioxidant compound sufficient to enhance collagen synthesis can be applied topically and penetrate the skin; (3) a mixture of essential amino acids; (4) a supplemental source of sulfur; and (5) a topical pharmaceutically acceptable carrier. The antioxidant compound can be lipoic acid, a lipoic acid analogue or derivative, a bioflavonoid, a constituent of ginkgo, or an isoflavone. The organic penetrant is preferably benzyl alcohol. Other ingredients, such as esters of tocopherol and ascorbic acid, can be included.
Owner:NIMNI MARCEL +1
Artificial cartilage tissue and production method thereof
ActiveUS20070184550A1Improve securityImprove portabilityBiocideArtificial cell constructsHeterologousCell-Extracellular Matrix
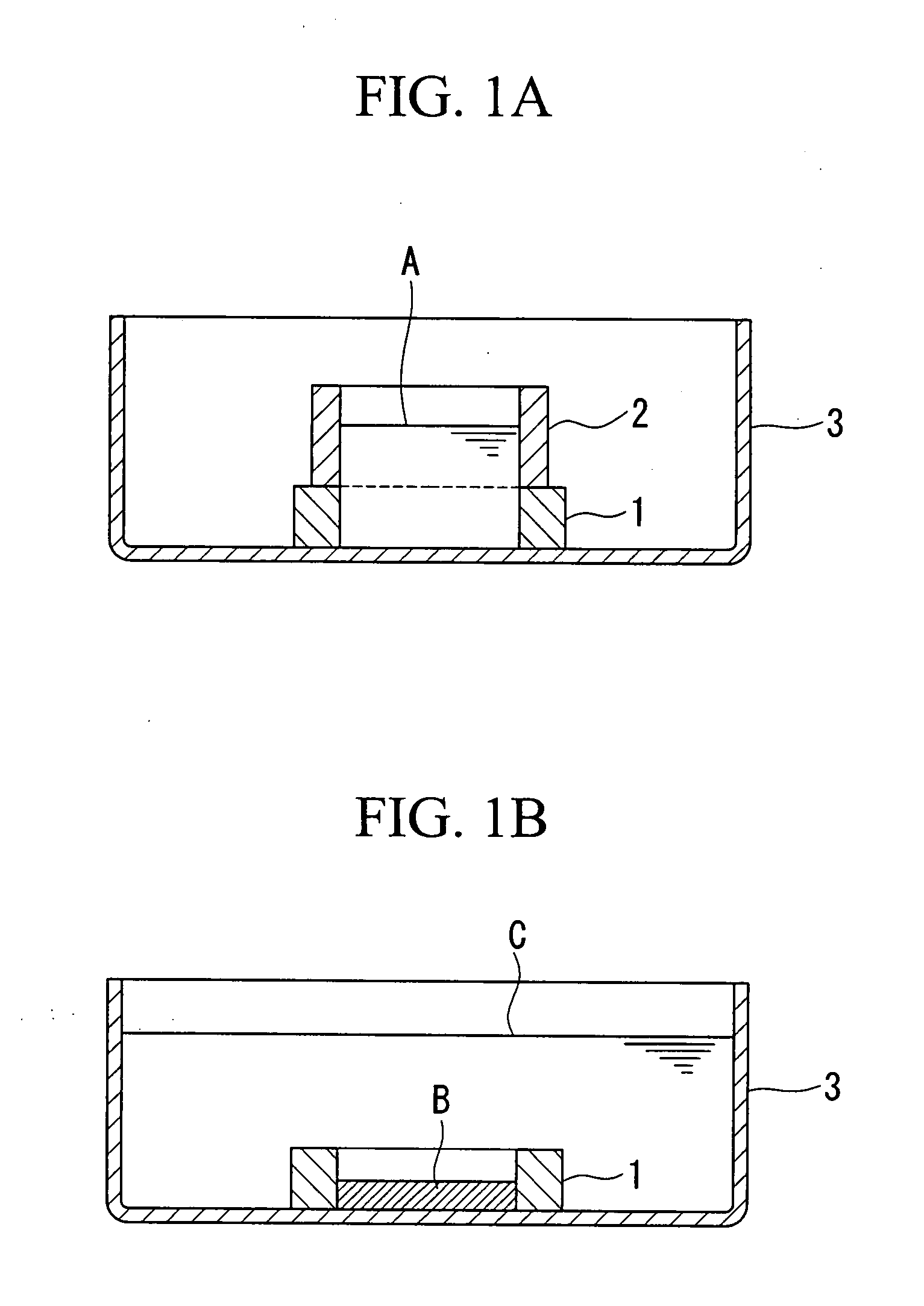
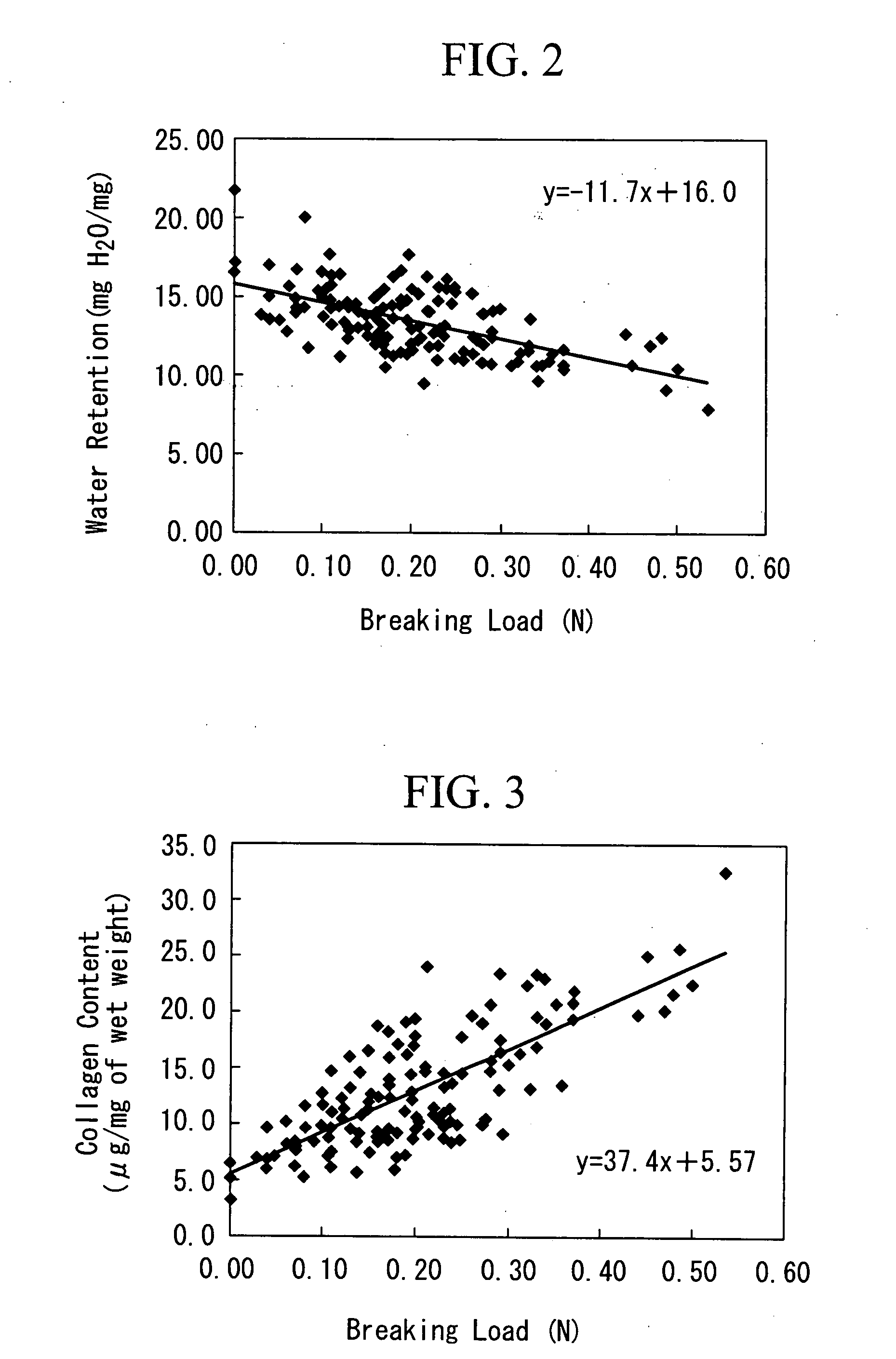
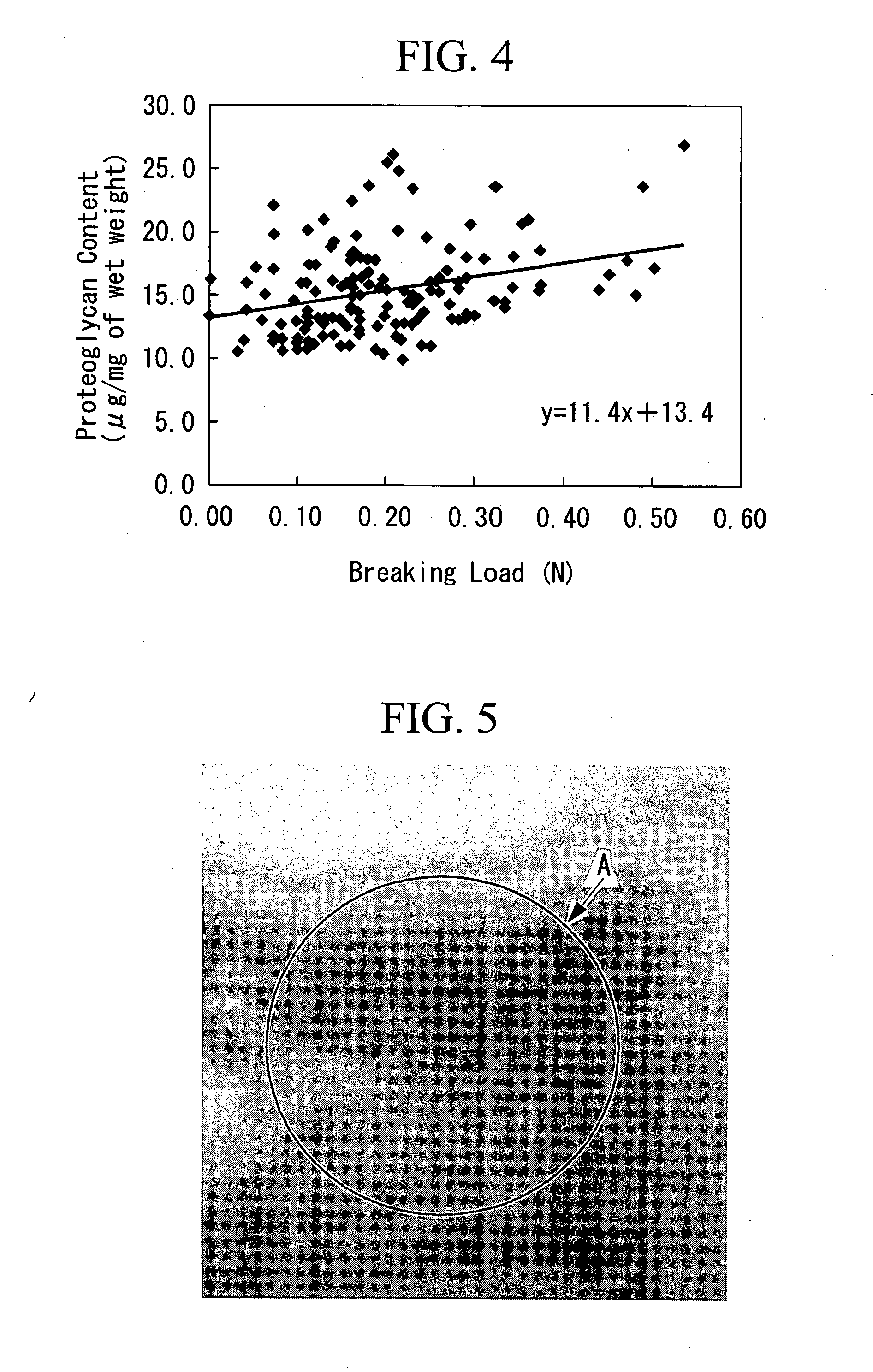
To obtain an artificial cartilage tissue having high strength and biocompatibility. There is provided an artificial cartilage tissue which is substantially free of heterologous protein and is substantially free of scaffold derived from non-cartilage-derived cells, but contains mammalian cartilage-derived cells and an extracellular matrix formed by the cartilage-derived cells, where: (1) the water retention per mg of tissue dry weight is 17.8 mg or less; (2) collagen of 5.57 μg or more per mg of tissue wet weight is contained; (3) proteoglycan of 9.22 μg or more per mg of tissue wet weight is contained.
Owner:PG RES
Modulators of EphA2 and EphrinA1 for the treatment of fibrosis-related disease
InactiveUS20060122138A1Maintaining organizationInhibit and decrease angiogenesisSenses disorderAntipyreticPsoriatic arthropathyDiabetic retinopathy
The present invention relates to methods and compositions designed for the treatment, management, prevention and / or amelioration of non-neoplastic hyperproliferative epithelial and / or endothelial cell disorders, including but not limited to disorders associated with increased deposition of extracellular matrix components (e.g., collagen, proteoglycans, tenascin and fibronectin) and / or aberrant angiogenesis. Non-limiting examples of such disorders include cirrhosis, fibrosis (e.g., fibrosis of the liver, kidney, lungs, heart, retina and other viscera), asthma, ischemia, atherosclerosis, diabetic retinopathy, retinopathy of prematurity, vascular restenosis, macular degeneration, rheumatoid arthritis, osteoarthritis, infantile hemangioma, verruca vulgaris, Kaposi's sarcoma, neurofibromatosis, recessive dystrophic epidermolysis bullosa, ankylosing spondylitis, systemic lupus, Reiter's syndrome, Sjogren's syndrome, endometriosis, preeclampsia, atherosclerosis, coronary artery disease, psoriatic arthropathy and psoriasis. The methods of the invention comprise the administration of an effective amount of one or more agents that are modulators of EphA2 and / or its endogenous ligand, EphrinA1. The invention also provides pharmaceutical compositions comprising one or more EphA2 / EphrinA1 Modulators of the invention either alone or in combination with one or more other agents useful for therapy for such non-neoplastic hyperproliferative epithelial and / or endothelial disorders. Diagnostic methods and methods for screening for EphA2 / EphrinA1 Modulators are also provided.
Owner:MEDIMMUNE LLC
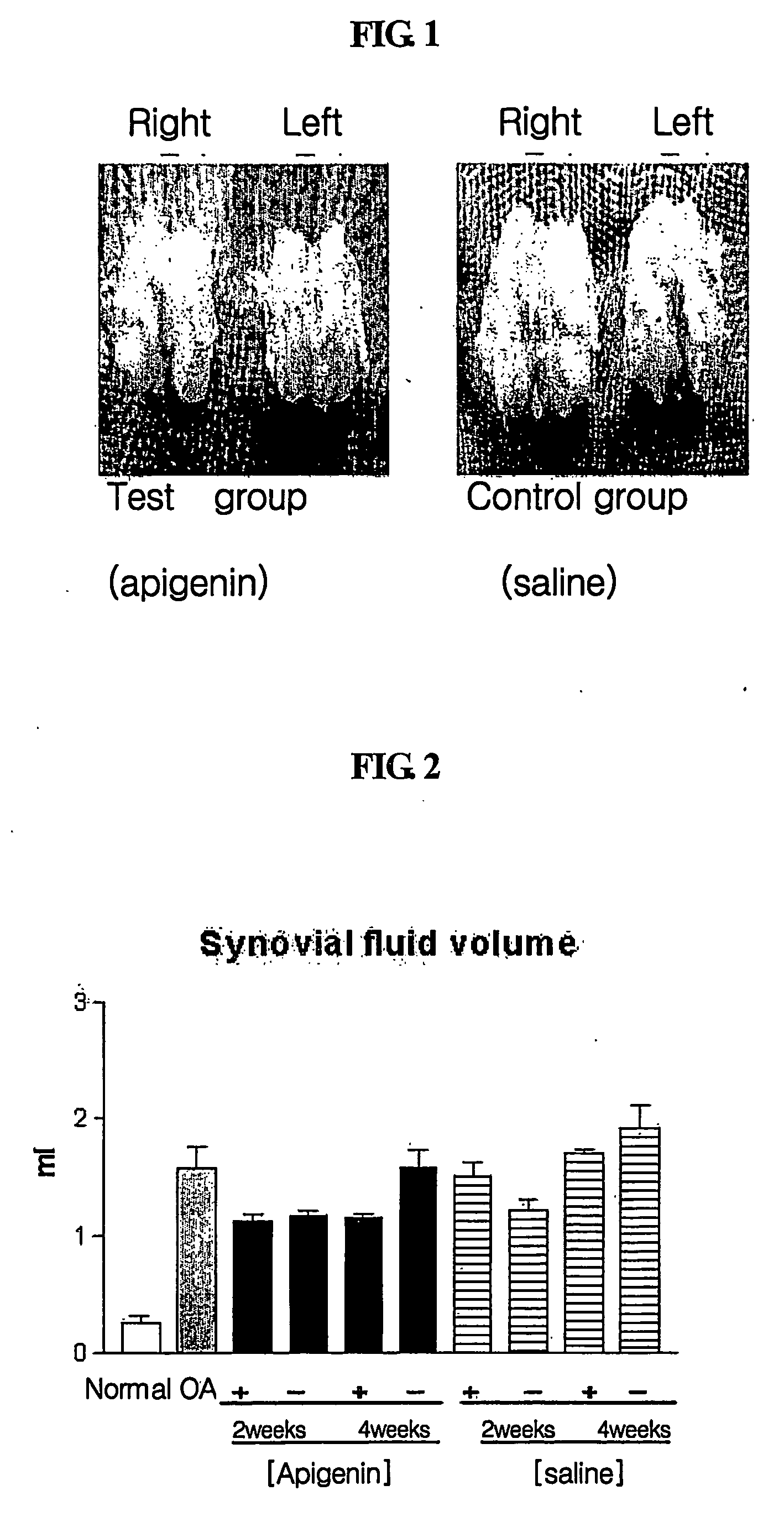
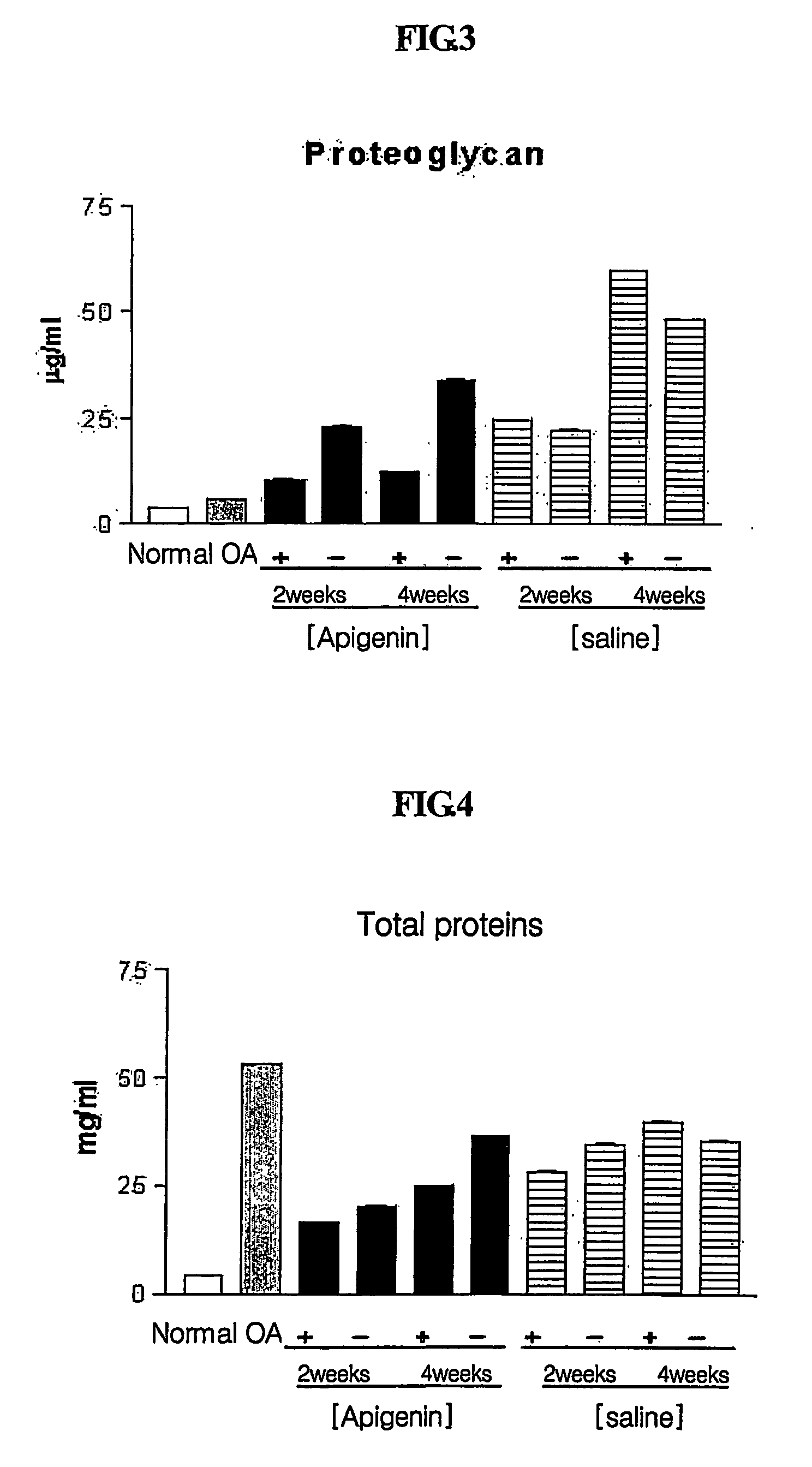
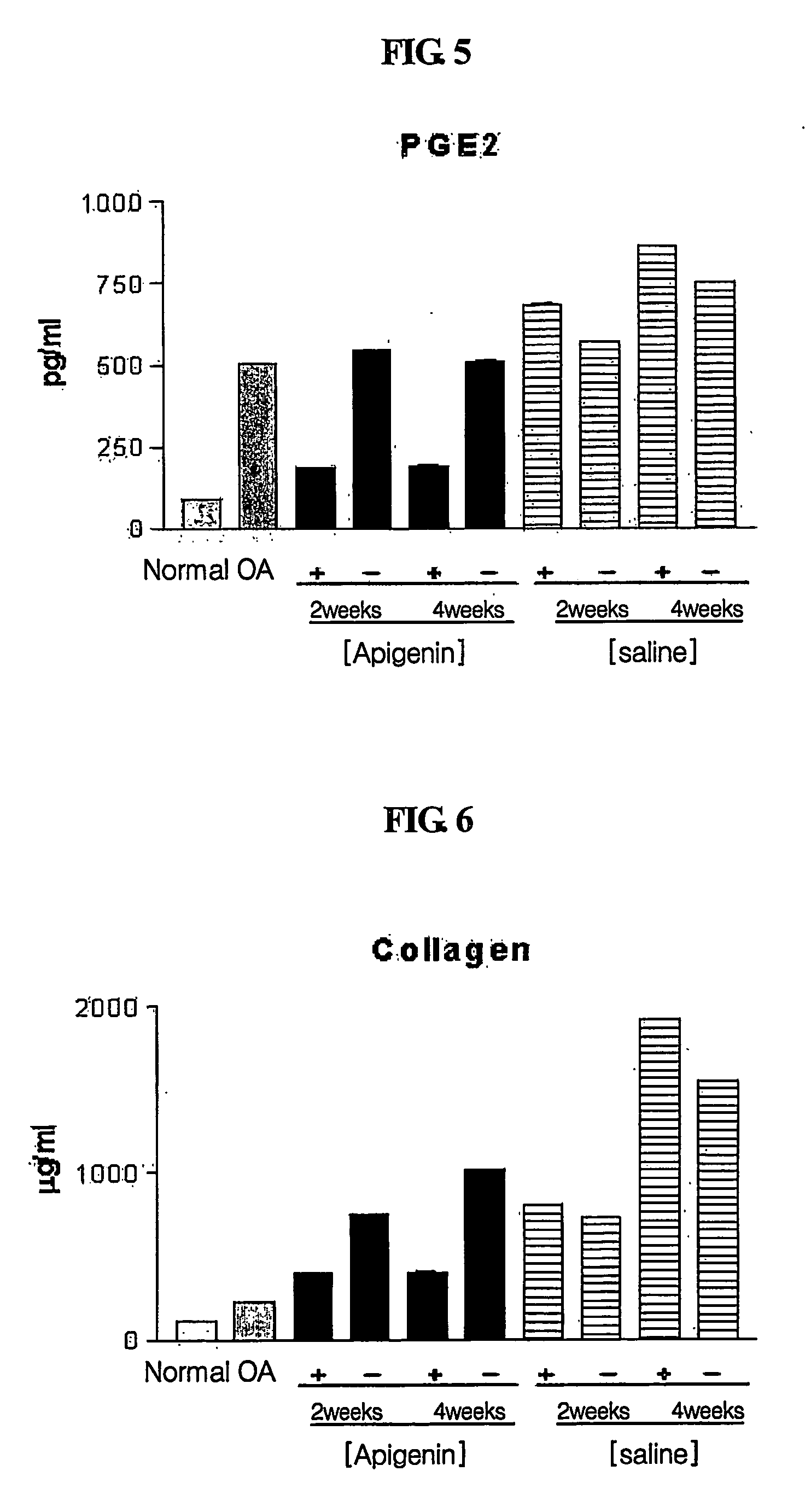
Composition for treatment of osteoarthritis containing apigenin as chondroregenerative agent
InactiveUS20070154540A1Good anti-inflammatory effectIncrease the amount of fluidBiocideAntipyreticApigeninSynovial Cell
Disclosed is a novel use of apigenin as a chondroregenerative agent, which has the effects of reducing elevated of cartilage destruction markers including total synovial fluid volume and proteoglycan, total proteins and prostaglandin in a synovial fluid, improving the condition of synovial cells, and regenerating cartilage. Also, the present invention discloses a therapeutic agent for osteoarthritis comprising apigenin as an agent regenerating articular cartilage, and a method of treating osteoarthritis using such a therapeutic agent.
Owner:YUHAN
Shark cartilage extracts and use thereof for immunomodulation
InactiveUS20050175711A1Stimulating cellularStimulating humoral componentAntibacterial agentsBiocideImmunomodulationsUronic acid
Disclosed is a composition from shark cartilage comprising immunoactive proteoglycans and other immunoactive components having a molecular weight greater than about 100 KD, wherein the composition has a uronic acid content of about 0.05-0.5 μg glucuronic acid per μg of the composition. The composition may be useful for treating or preventing tumor growth, bacterial infections, viral infections and / or fungal infections.
Owner:OCEAN NUTRITION CANADA
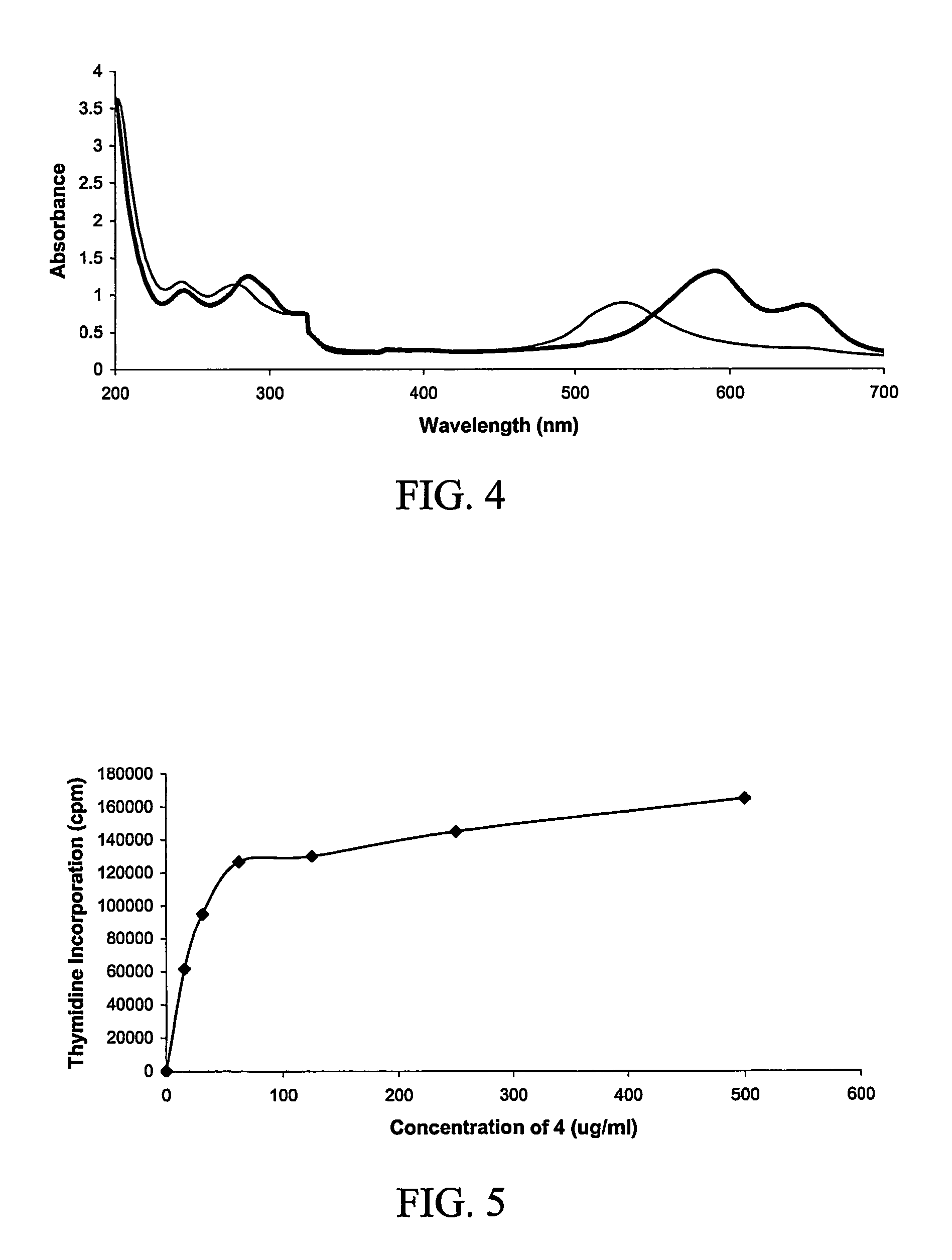
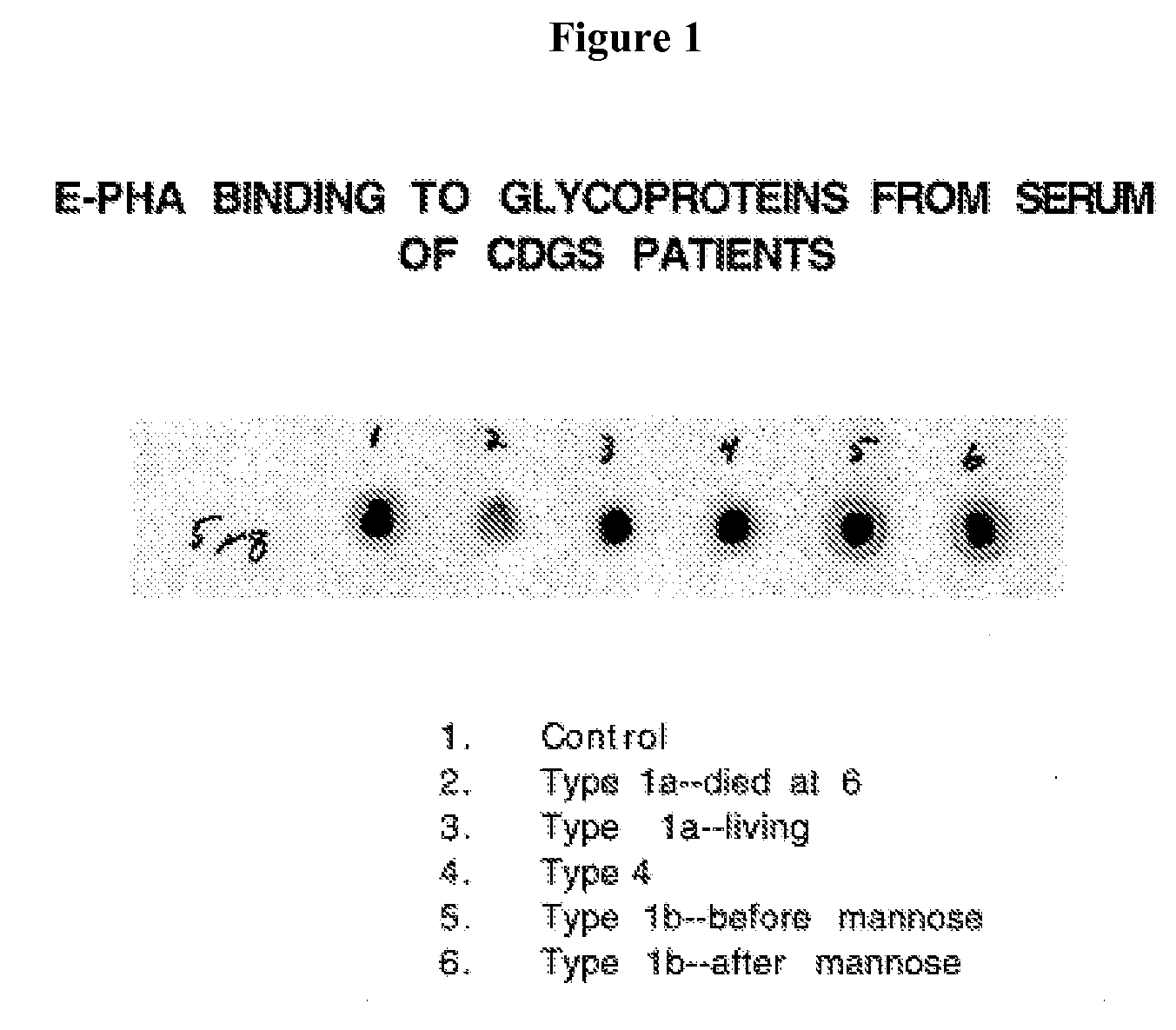
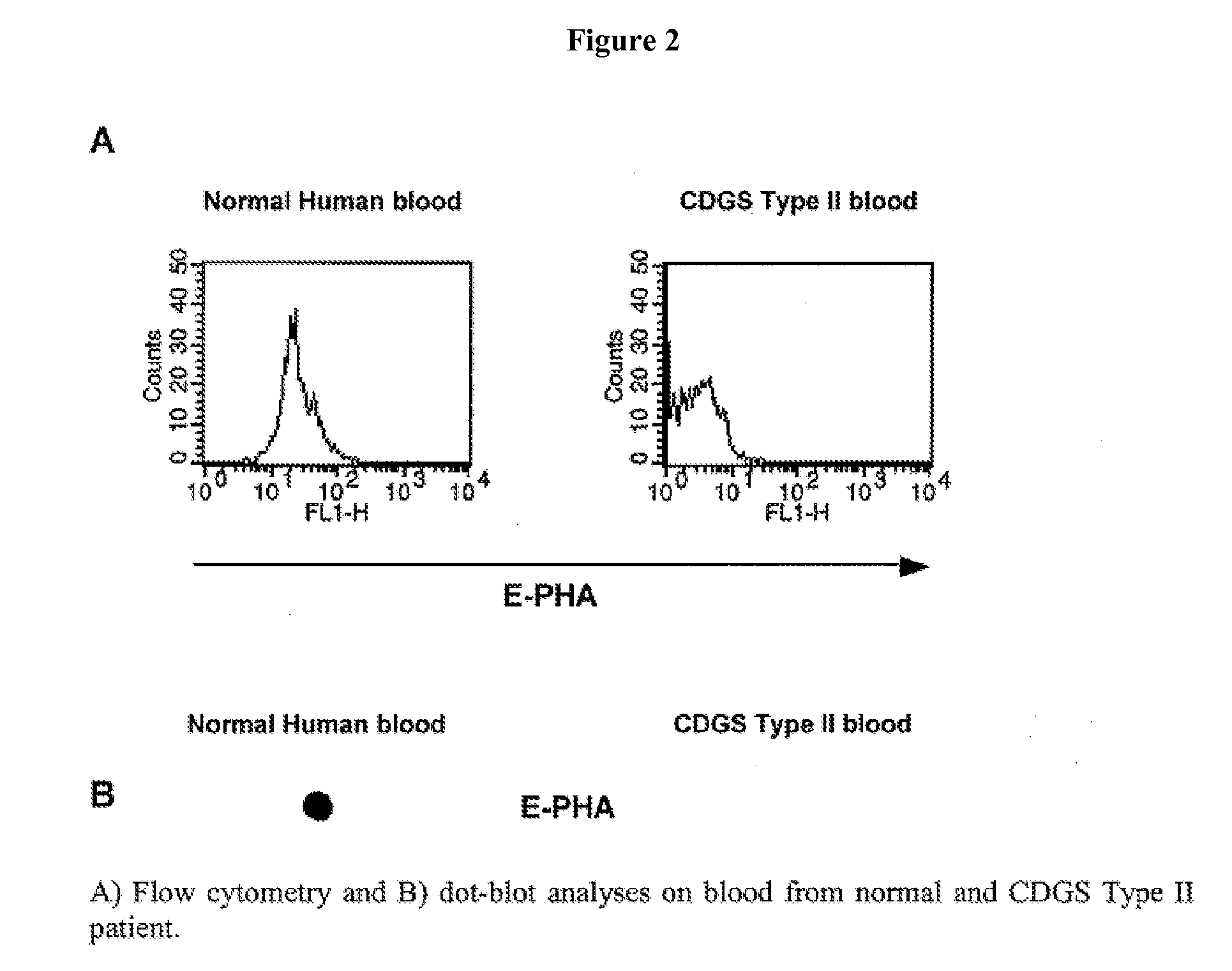
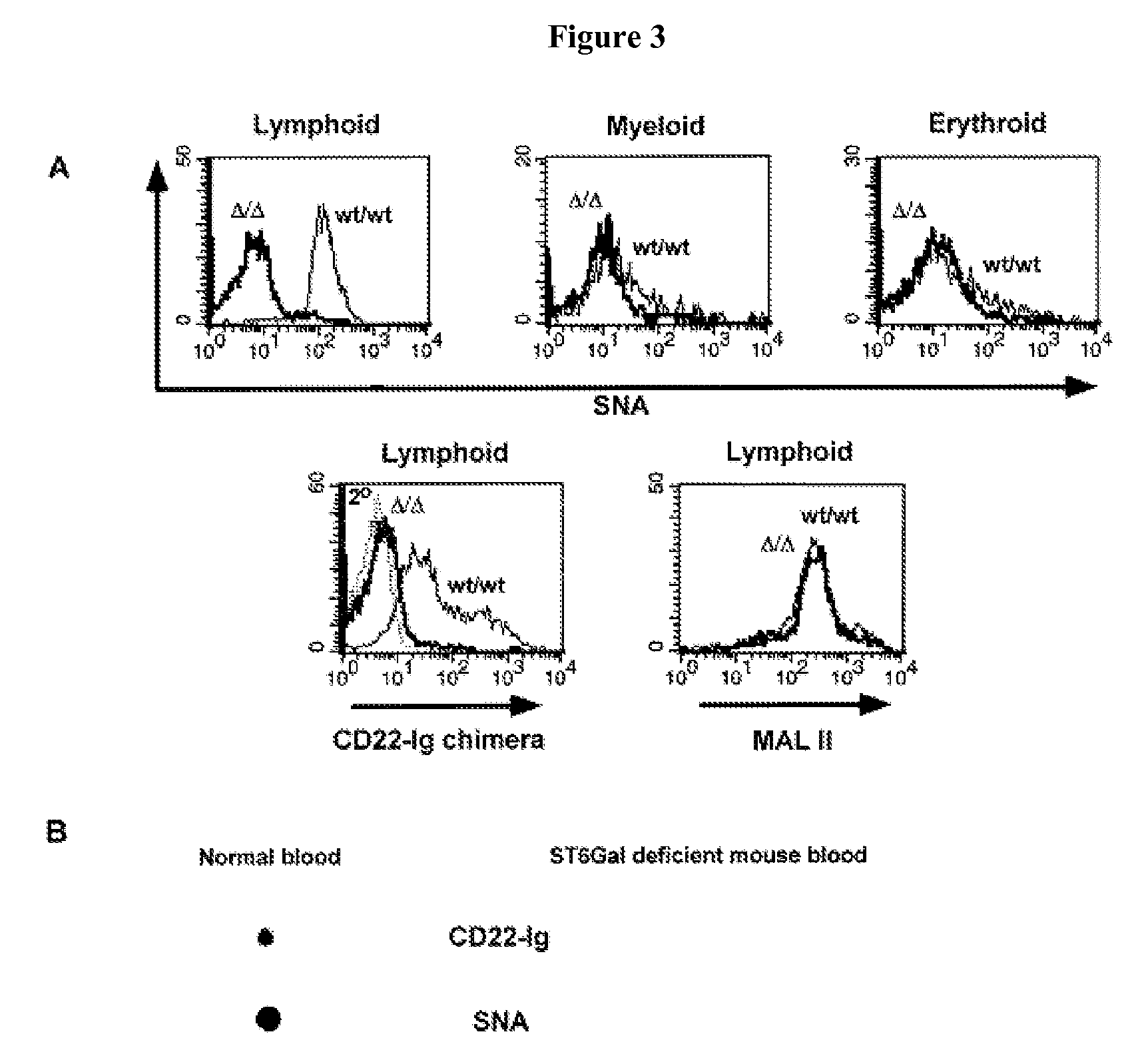
Diagnosis of human glycosylation disorders
InactiveUS20060228762A1Microbiological testing/measurementTransferasesGlycoconjugateTransmitted disease
This invention provides methods and kits for use in diagnosing genetically transmitted diseases that are associated with deficiencies in glycosylation of glycoconjugates such as glycoproteins, glycolipids, and proteoglycans. The methods and kits are also useful for monitoring the course of treatment of diseases that are associated with glycosylation disorders.
Owner:RGT UNIV OF CALIFORNIA +1
Methods of proliferating stem cells
InactiveUS20090137038A1Loss of multipotentialityIncreased proliferationBiocideDead animal preservationMultipotentialityGlycosaminoglycan
The invention relates to methods of proliferating stem cells. More particularly, the invention relates to the use of glycosaminoglycans or proteoglycans to promote the growth of stem cells in ex vivo culture, while preserving their multipotentiality.
Owner:AGENCY FOR SCI TECH & RES
Proteoglycan isolated from cartilaginous fish and process for producing the same
InactiveUS20070010430A1High activityIncrease volumeSenses disorderAntipyreticAlcoholEffective action
There are provided a cartilage extract that can be effective even when taken in a small amount and has a clear mechanism of effective action and a method of producing the extract. Provided are an isolated proteoglycan which is derived from a water extract of cartilage of cartilaginous fish and whose main component has a molecular weight of 500 kDa or more; a composition containing the proteoglycan; a pharmaceutical composition containing the proteoglycan as an active ingredient; and a method of producing the proteoglycan, comprising the steps of pulverizing cartilaginous fish-derived cartilage into a pulverized product with an average particle diameter of 100 μm or less, adding water to the pulverized product and extracting water-soluble components from it, separating an aqueous phase that contains the extracted water-soluble components, and adding an alcohol to the aqueous phase to produce a precipitate.
Owner:HOSOKAWA MICRON CORP
Decellularized Biomaterial from Non-Mammalian Tissue
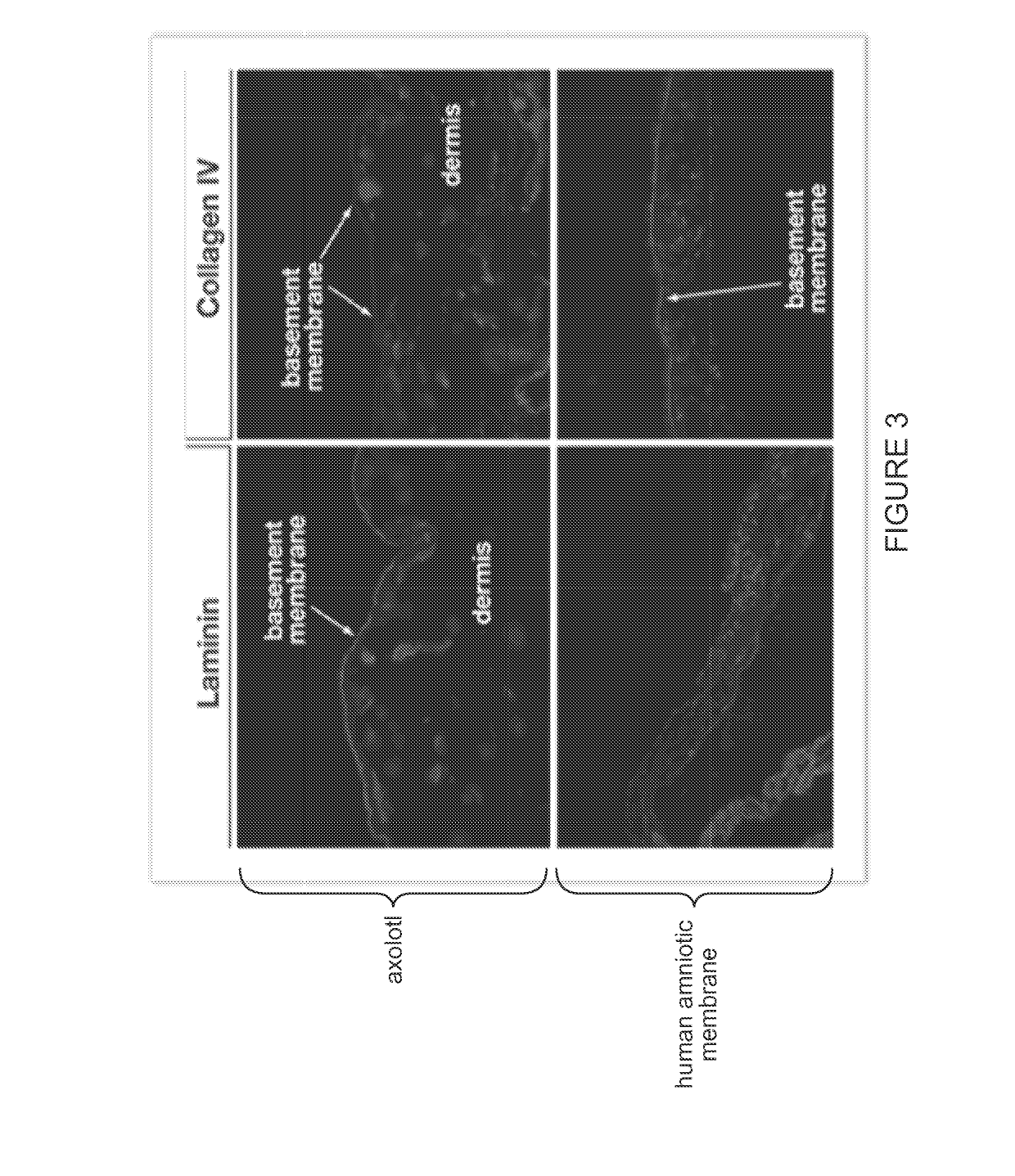
InactiveUS20190255217A1Lower immune responseReduce inflammationAmphibian material medical ingredientsPeptide/protein ingredientsCellular componentFunctional integrity
The growth factor profile, connective tissue matrix constituents, and immunoprivileged status of urodele extracellular matrix (ECM) and accompanying cutaneous tissue, plus the presence of antimicrobial peptides there, render urodele-derived tissue an ideal source for biological scaffolds for xenotransplantation. In particular, a biological scaffold biomaterial can be obtained by a process that entails (A) obtaining a tissue sample from a urodele, where the tissue comprises ECM, inclusive of the basement membrane, and (B) subjecting the tissue sample to a decellularization process that maintains the structural and functional integrity of the extracellular matrix, by virtue of retaining its fibrous and non-fibrous proteins, glycoaminoglycans (GAGs) and proteoglycans, while removing sufficient cellular components of the sample to reduce or eliminate antigenicity and immunogenicity for xenograft purposes. The resultant urodele-derived biomaterial can be used to enhance restoration of skin homeostasis, to reduce the severity, duration and associated damage caused by post-surgical inflammation, and to promote progression of natural healing and regeneration processes. In addition, the biomaterial promotes the formation of remodeled tissue that is comparable in quality, function, and compliance to undamaged human tissue.
Owner:ISE PROFESSIONAL TESTING & CONSULTING SERVICES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com